|
Autocatalytic
A single chemical reaction is said to be autocatalytic if one of the reaction products is also a catalyst for the same or a coupled reaction.Steinfeld J.I., Francisco J.S. and Hase W.L. ''Chemical Kinetics and Dynamics'' (2nd ed., Prentice-Hall 1999) p.151-2 Such a reaction is called an autocatalytic reaction. A ''set'' of chemical reactions can be said to be "collectively autocatalytic" if a number of those reactions produce, as reaction products, catalysts for enough of the other reactions that the entire set of chemical reactions is self-sustaining given an input of energy and food molecules (see autocatalytic set). Chemical reactions A chemical reaction of two reactants and two products can be written as : \alpha A + \beta B \rightleftharpoons \sigma S + \tau T where the Greek letters are stoichiometric coefficients and the capital Latin letters represent chemical species. The chemical reaction proceeds in both the forward and reverse direction. This equation is easily g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Autocatalytic Set
An autocatalytic set is a collection of entities, each of which can be created catalytically by other entities within the set, such that as a whole, the set is able to catalyze its own production. In this way the set ''as a whole'' is said to be autocatalytic. Autocatalytic sets were originally and most concretely defined in terms of molecular entities, but have more recently been metaphorically extended to the study of systems in sociology, ecology, and economics. Autocatalytic sets also have the ability to replicate themselves if they are split apart into two physically separated spaces. Computer models illustrate that split autocatalytic sets will reproduce all of the reactions of the original set in each half, much like cellular mitosis. In effect, using the principles of autocatalysis, a small metabolism can replicate itself with very little high level organization. This property is why autocatalysis is a contender as the foundational mechanism for complex evolution. Prio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Logistic Function
A logistic function or logistic curve is a common S-shaped curve (sigmoid curve) with equation f(x) = \frac, where For values of x in the domain of real numbers from -\infty to +\infty, the S-curve shown on the right is obtained, with the graph of f approaching L as x approaches +\infty and approaching zero as x approaches -\infty. The logistic function finds applications in a range of fields, including biology (especially ecology), biomathematics, chemistry, demography, economics, geoscience, mathematical psychology, probability, sociology, political science, linguistics, statistics, and artificial neural networks. A generalization of the logistic function is the hyperbolastic function of type I. The standard logistic function, where L=1,k=1,x_0=0, is sometimes simply called ''the sigmoid''. It is also sometimes called the ''expit'', being the inverse of the logit. History The logistic function was introduced in a series of three papers by Pierre François Verhulst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Induction Period
An induction period in chemical kinetics is an initial slow stage of a chemical reaction; after the induction period, the reaction accelerates. Ignoring induction periods can lead to runaway reactions. In some catalytic reactions, a pre-catalyst needs to undergo a transformation to form the active catalyst, before the catalyst can take effect. Time is required for this transformation, hence the induction period. For example, with Wilkinson's catalyst, one triphenylphosphine ligand must dissociate to give the coordinatively unsaturated 14-electron species which can participate in the catalytic cycle: : Similarly, for an autocatalytic reaction, where one of the reaction products catalyzes the reaction itself, the rate of reaction is low initially until sufficient products have formed to catalyze the reaction. Reactions generally accelerate when heat is applied. Where a reaction is exothermic, the rate of the reaction may initially be low. As the reaction proceeds, heat is generat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
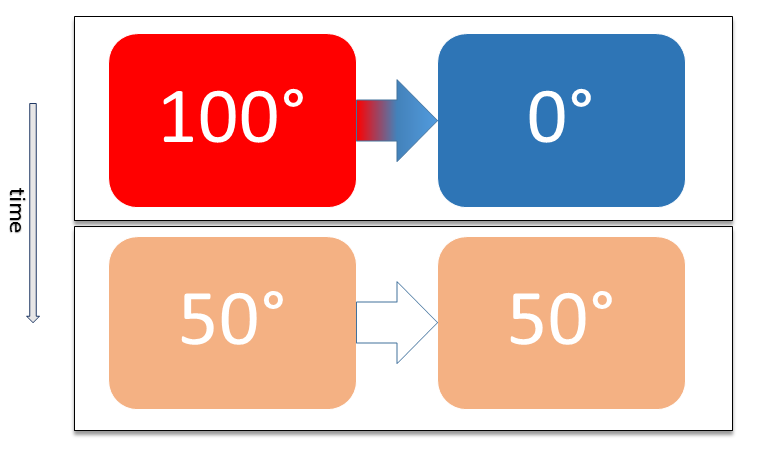
Thermal Equilibrium
Two physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be in thermal equilibrium with itself if the temperature within the system is spatially uniform and temporally constant. Systems in thermodynamic equilibrium are always in thermal equilibrium, but the converse is not always true. If the connection between the systems allows transfer of energy as 'change in internal energy' but does not allow transfer of matter or transfer of energy as work, the two systems may reach thermal equilibrium without reaching thermodynamic equilibrium. Two varieties of thermal equilibrium Relation of thermal equilibrium between two thermally connected bodies The relation of thermal equilibrium is an instance of equilibrium between two bodies, which means that it refers to transfer through a selectively permeable p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vortex
In fluid dynamics, a vortex ( : vortices or vortexes) is a region in a fluid in which the flow revolves around an axis line, which may be straight or curved. Vortices form in stirred fluids, and may be observed in smoke rings, whirlpools in the wake of a boat, and the winds surrounding a tropical cyclone, tornado or dust devil. Vortices are a major component of turbulent flow. The distribution of velocity, vorticity (the curl of the flow velocity), as well as the concept of circulation are used to characterise vortices. In most vortices, the fluid flow velocity is greatest next to its axis and decreases in inverse proportion to the distance from the axis. In the absence of external forces, viscous friction within the fluid tends to organise the flow into a collection of irrotational vortices, possibly superimposed to larger-scale flows, including larger-scale vortices. Once formed, vortices can move, stretch, twist, and interact in complex ways. A moving vortex carries s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hurricane
A tropical cyclone is a rapidly rotating storm system characterized by a low-pressure center, a closed low-level atmospheric circulation, strong winds, and a spiral arrangement of thunderstorms that produce heavy rain and squalls. Depending on its location and strength, a tropical cyclone is referred to by different names, including hurricane (), typhoon (), tropical storm, cyclonic storm, tropical depression, or simply cyclone. A hurricane is a strong tropical cyclone that occurs in the Atlantic Ocean or northeastern Pacific Ocean, and a typhoon occurs in the northwestern Pacific Ocean. In the Indian Ocean, South Pacific, or (rarely) South Atlantic, comparable storms are referred to simply as "tropical cyclones", and such storms in the Indian Ocean can also be called "severe cyclonic storms". "Tropical" refers to the geographical origin of these systems, which form almost exclusively over tropical seas. "Cyclone" refers to their winds moving in a circle, whirling round ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emergence
In philosophy, systems theory, science, and art, emergence occurs when an entity is observed to have properties its parts do not have on their own, properties or behaviors that emerge only when the parts interact in a wider whole. Emergence plays a central role in theories of integrative levels and of complex systems. For instance, the phenomenon of life as studied in biology is an emergent property of chemistry. In philosophy, theories that emphasize emergent properties have been called emergentism. In philosophy Philosophers often understand emergence as a claim about the etiology of a system's properties. An emergent property of a system, in this context, is one that is not a property of any component of that system, but is still a feature of the system as a whole. Nicolai Hartmann (1882–1950), one of the first modern philosophers to write on emergence, termed this a ''categorial novum'' (new category). Definitions This concept of emergence dates from at least the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Bath
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is also often used to refer to the thermal energy contained in a system as a component of its internal energy and that is reflected in the temperature of the system. For both uses of the term, heat is a form of energy. An example of formal vs. informal usage may be obtained from the right-hand photo, in which the metal bar is "conducting heat" from its hot end to its cold end, but if the metal bar is considered a thermodynamic system, then the energy flowing within the metal bar is called internal energy, not heat. The hot metal bar is also transferring heat to its surroundings, a correct statement for both the strict and loose meanings of ''heat''. Another example of informal usage is the term '' heat content'', used despite the fact that ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Random
In common usage, randomness is the apparent or actual lack of pattern or predictability in events. A random sequence of events, symbols or steps often has no :wikt:order, order and does not follow an intelligible pattern or combination. Individual random events are, by definition, unpredictable, but if the probability distribution is known, the frequency of different outcomes over repeated events (or "trials") is predictable.Strictly speaking, the frequency of an outcome will converge almost surely to a predictable value as the number of trials becomes arbitrarily large. Non-convergence or convergence to a different value is possible, but has probability zero. For example, when throwing two dice, the outcome of any particular roll is unpredictable, but a sum of 7 will tend to occur twice as often as 4. In this view, randomness is not haphazardness; it is a measure of uncertainty of an outcome. Randomness applies to concepts of chance, probability, and information entropy. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Closed System
A closed system is a natural physical system that does not allow transfer of matter in or out of the system, although — in contexts such as physics, chemistry or engineering — the transfer of energy (''e.g.'' as work or heat) is allowed. In physics In classical mechanics In nonrelativistic classical mechanics, a closed system is a physical system that doesn't exchange any matter with its surroundings, and isn't subject to any net force whose source is external to the system. A closed system in classical mechanics would be equivalent to an isolated system in thermodynamics. Closed systems are often used to limit the factors that can affect the results of a specific problem or experiment. In thermodynamics In thermodynamics, a closed system can exchange energy (as heat or work) but not matter, with its surroundings. An isolated system cannot exchange any heat, work, or matter with the surroundings, while an open system can exchange energy and matter. (This scheme of definit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change, and information systems including the transmission of information in telecommunication. The thermodynamic concept was referred to by Scottish scientist and engineer William Rankine in 1850 with the names ''thermodynamic function'' and ''heat-potential''. In 1865, German physicist Rudolf Clausius, one of the leading founders of the field of thermodynamics, defined it as the quotient of an infinitesimal amount of hea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |