|
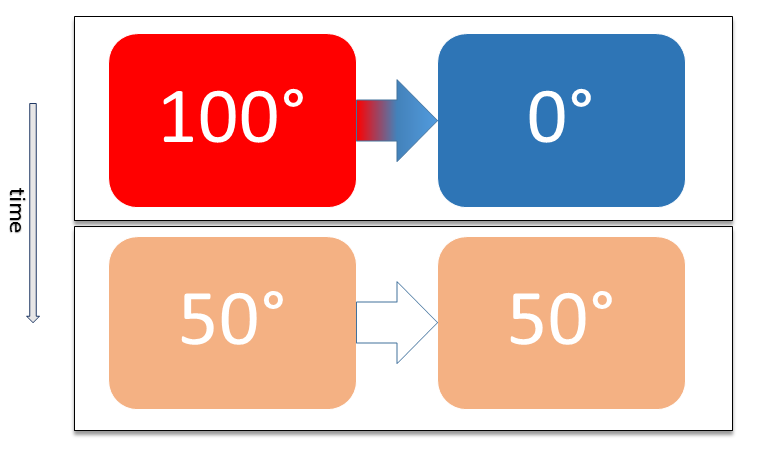
Thermal Equilibrium
Two physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be in thermal equilibrium with itself if the temperature within the system is spatially uniform and temporally constant. Systems in thermodynamic equilibrium are always in thermal equilibrium, but the converse is not always true. If the connection between the systems allows transfer of energy as 'change in internal energy' but does not allow transfer of matter or transfer of energy as work, the two systems may reach thermal equilibrium without reaching thermodynamic equilibrium. Two varieties of thermal equilibrium Relation of thermal equilibrium between two thermally connected bodies The relation of thermal equilibrium is an instance of equilibrium between two bodies, which means that it refers to transfer through a selectively permeable p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Equilibrium In Closed System
A thermal column (or thermal) is a rising mass of buoyant air, a convective current in the atmosphere, that transfers heat energy vertically. Thermals are created by the uneven heating of Earth's surface from solar radiation, and are an example of convection, specifically atmospheric convection. Thermals on Earth The Sun warms the ground, which in turn warms the air directly above. The warm air near the surface expands, becoming less dense than the surrounding air. The lighter air rises and cools due to its expansion in the lower pressure at higher altitudes. It stops rising when it has cooled to the same temperature, thus density, as the surrounding air. Associated with a thermal is a downward flow surrounding the thermal column. The downward-moving exterior is caused by colder air being displaced at the top of the thermal. The size and strength of thermals are influenced by the properties of the lower atmosphere (the ''troposphere''). When the air is cold, bubbles of warm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adiabatic Wall
In thermodynamics, an adiabatic wall between two thermodynamic systems does not allow heat or chemical substances to pass across it, in other words there is no heat transfer or mass transfer. In theoretical investigations, it is sometimes assumed that one of the two systems is the surroundings of the other. Then it is assumed that the work transferred is reversible within the surroundings, but in thermodynamics it is not assumed that the work transferred is reversible within the system. The assumption of reversibility in the surroundings has the consequence that the quantity of work transferred is well defined by macroscopic variables in the surroundings. Accordingly, the surroundings are sometimes said to have a reversible work reservoir. Along with the idea of an adiabatic wall is that of an adiabatic enclosure. It is easily possible that a system has some boundary walls that are adiabatic and others that are not. When some are not adiabatic, then the system is not adiabatically e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sydney Chapman (mathematician)
Sydney Chapman (29 January 1888 – 16 June 1970) was a British mathematician and geophysicist. His work on the kinetic theory of gases, solar-terrestrial physics, and the Earth's ozone layer has inspired a broad range of research over many decades. Education and early life Chapman was born in Eccles, near Salford in England and began his advanced studies at a technical institute, now the University of Salford, in 1902. In 1904 at age 16, Chapman entered the University of Manchester. He competed for a scholarship to the university offered by his home county, and was the last student selected. Chapman later reflected, "I sometimes wonder what would have happened if I'd hit one place lower." He initially studied engineering in the department headed by Osborne Reynolds. Chapman was taught mathematics by Horace Lamb, the Beyer professor of mathematics, and J. E. Littlewood, who came from Cambridge in Chapman's final year at Manchester. Although he graduated with an engineerin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ludwig Boltzmann
Ludwig Eduard Boltzmann (; 20 February 1844 – 5 September 1906) was an Austrian physicist and philosopher. His greatest achievements were the development of statistical mechanics, and the statistical explanation of the second law of thermodynamics. In 1877 he provided the current definition of entropy, S = k_ \ln \Omega \!, where Ω is the number of microstates whose energy equals the system's energy, interpreted as a measure of statistical disorder of a system. Max Planck named the constant the Boltzmann constant. Statistical mechanics is one of the pillars of modern physics. It describes how macroscopic observations (such as temperature and pressure) are related to microscopic parameters that fluctuate around an average. It connects thermodynamic quantities (such as heat capacity) to microscopic behavior, whereas, in classical thermodynamics, the only available option would be to measure and tabulate such quantities for various materials. Biography Childhood and educatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Oscillator
A thermal oscillator is a system where conduction along thermal gradients overshoots thermal equilibrium, resulting in thermal oscillations where parts of the system oscillate between being colder and hotter than average. References {{Reflist Thermodynamics ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiative Equilibrium
Radiative equilibrium is the condition where the total thermal radiation leaving an object is equal to the total thermal radiation entering it. It is one of the several requirements for thermodynamic equilibrium, but it can occur in the absence of thermodynamic equilibrium. There are various types of radiative equilibrium, which is itself a kind of dynamic equilibrium. Definitions Equilibrium, in general, is a state in which opposing forces are balanced, and hence a system does not change in time. Radiative equilibrium is the specific case of thermal equilibrium, for the case in which the exchange of heat is done by radiative heat transfer. There are several types of radiative equilibrium. Prevost's definitions An important early contribution was made by Pierre Prevost in 1791. Prevost considered that what is nowadays called the photon gas or electromagnetic radiation was a fluid that he called "free heat". Prevost proposed that free radiant heat is a very rare fluid, rays of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Equilibrium
Thermodynamic equilibrium is an axiomatic concept of thermodynamics. It is an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable walls. In thermodynamic equilibrium, there are no net macroscopic flows of matter nor of energy within a system or between systems. In a system that is in its own state of internal thermodynamic equilibrium, no macroscopic change occurs. Systems in mutual thermodynamic equilibrium are simultaneously in mutual thermal, mechanical, chemical, and radiative equilibria. Systems can be in one kind of mutual equilibrium, while not in others. In thermodynamic equilibrium, all kinds of equilibrium hold at once and indefinitely, until disturbed by a thermodynamic operation. In a macroscopic equilibrium, perfectly or almost perfectly balanced microscopic exchanges occur; this is the physical explanation of the notion of macroscopic equilibrium. A thermodynamic sys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around 1 millimeter (300 GHz) to the nominal red edge of the visible spectrum, around 700 nanometers (430 THz). Longer IR wavelengths (30 μm-100 μm) are sometimes included as part of the terahertz radiation range. Almost all black-body radiation from objects near room temperature is at infrared wavelengths. As a form of electromagnetic radiation, IR propagates energy and momentum, exerts radiation pressure, and has properties corresponding to both those of a wave and of a particle, the photon. It was long known that fires emit invisible heat; in 1681 the pioneering experimenter Edme Mariotte showed that glass, though transparent to sunlight, obstructed radiant heat. In 1800 the astronomer Sir William Herschel discovered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Irradiance
Solar irradiance is the power per unit area (surface power density) received from the Sun in the form of electromagnetic radiation in the wavelength range of the measuring instrument. Solar irradiance is measured in watts per square metre (W/m2) in SI units. Solar irradiance is often integrated over a given time period in order to report the radiant energy emitted into the surrounding environment (joule per square metre, J/m2) during that time period. This integrated solar irradiance is called solar irradiation, solar exposure, solar insolation, or insolation. Irradiance may be measured in space or at the Earth's surface after atmospheric absorption and scattering. Irradiance in space is a function of distance from the Sun, the solar cycle, and cross-cycle changes.Michael Boxwell, ''Solar Electricity Handbook: A Simple, Practical Guide to Solar Energy'' (2012), p. 41–42. Irradiance on the Earth's surface additionally depends on the tilt of the measuring surface, the hei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Harald Wergeland
Harald Nicolai Storm Wergeland (14 March 1912 – 25 January 1987) was a Norwegian physicist. He was a professor at the Norwegian Institute of Technology. He was born in Norderhov as a son of forest manager Harald Nicolay Storm Wergeland (1884–1953) and Ebba Marie Weien (1889–1952). In 1937 he married Hedvig Louise Ording, a sister of Fredrik Ording. He finished his secondary education in 1931. He graduated as a chemical engineer from the Norwegian Institute of Technology in 1936 and earned the dr.philos. degree in 1942. He was briefly a teacher at Trondheim Commerce School before working as an assistant at the Norwegian Institute of Technology from 1939. Wergeland worked as a professor of physics from 1946 to 1979 at the Norwegian Institute of Technology, now the Norwegian University of Science and Technology at Gløshaugen. He also served as associate professor at Purdue University from 1948 to 1949. Wergeland participated in the foundation of CERN and was a leading m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dirk Ter Haar
Dirk ter Haar FRSE FIP DSc (; Oosterwolde, 19 April 1919 – Drachten, 3 September 2002) was an Anglo-Dutch physicist. Life Dirk ter Haar was born at Oosterwolde in the province Friesland in the north of the Netherlands on 19 April 1919. He studied physics at the University of St Andrews in Scotland and Oxford University in England, obtaining an MA degree before carrying out postgraduate studies at Leiden University. In 1946 he was a research fellow of Niels Bohr at the Institute for Theoretical Physics in Copenhagen (now the Niels Bohr Institute), and received his PhD in Leiden in 1948 from Hendrik Kramers for a dissertation on the origin of the Solar System. From 1947 to 1950 he was a visiting associate professor of physics at Purdue University. In 1950 he obtained a post as professor of physics at the University of St. Andrews, and later became a British citizen. In 1952 he was elected a Fellow of the Royal Society of Edinburgh. His proposers were Jack Allen, David Jack, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Second Law Of Thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and Energy transformation, energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects (or "downhill"), unless energy in some form is supplied to reverse the direction of heat flow. Another definition is: "Not all heat energy can be converted into Work (thermodynamics), work in a cyclic process."Young, H. D; Freedman, R. A. (2004). ''University Physics'', 11th edition. Pearson. p. 764. The second law of thermodynamics in other versions establishes the concept of entropy as a physical property of a thermodynamic system. It can be used to predict whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. The second law may be formulated by the observation that the entropy of isolated systems ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |