Smoke From Cromwell's Time And Other Stories on:
[Wikipedia]
[Google]
[Amazon]



 Smoke is a suspension of airborne
Smoke is a suspension of airborne  Smoke inhalation is the primary cause of death in victims of indoor
Smoke inhalation is the primary cause of death in victims of indoor
 Pyrolysis of burning material, especially
Pyrolysis of burning material, especially

 The naked eye detects particle sizes greater than 7 µm (micrometres). Visibility, Visible particles emitted from a fire are referred to as smoke. Invisibility, Invisible particles are generally referred to as gas or fumes. This is best illustrated when Toast (food), toasting bread in a toaster. As the bread heats up, the products of combustion increase in size. The fumes initially produced are invisible but become visible if the toast is burnt.
An ionization chamber type smoke detector is technically a product of combustion detector, not a smoke detector. Ionization chamber type smoke detectors detect particles of combustion that are invisible to the naked eye. This explains why they may frequently false alarm from the fumes emitted from the red-hot heating elements of a toaster, before the presence of visible smoke, yet they may fail to activate in the early, low-heat
The naked eye detects particle sizes greater than 7 µm (micrometres). Visibility, Visible particles emitted from a fire are referred to as smoke. Invisibility, Invisible particles are generally referred to as gas or fumes. This is best illustrated when Toast (food), toasting bread in a toaster. As the bread heats up, the products of combustion increase in size. The fumes initially produced are invisible but become visible if the toast is burnt.
An ionization chamber type smoke detector is technically a product of combustion detector, not a smoke detector. Ionization chamber type smoke detectors detect particles of combustion that are invisible to the naked eye. This explains why they may frequently false alarm from the fumes emitted from the red-hot heating elements of a toaster, before the presence of visible smoke, yet they may fail to activate in the early, low-heat
 Cigarette smoke is a major modifiable risk factor for lung disease, heart disease, and many cancers. Smoke can also be a component of ambient air pollution due to the burning of coal in power plants, forest fires or other sources, although the concentration of pollutants in ambient air is typically much less than that in cigarette smoke. One day of exposure to PM2.5 at a concentration of 880 μg/m3, such as occurs in Beijing, China, is the equivalent of smoking one or two cigarettes in terms of particulate inhalation by weight. The analysis is complicated, however, by the fact that the organic compounds present in various ambient particulates may have a higher carcinogenicity than the compounds in cigarette smoke particulates. Secondhand tobacco smoke is the combination of both sidestream and mainstream smoke emissions from a burning tobacco product. These emissions contain more than 50 carcinogenic chemicals. According to the United States Surgeon General of the United States, Surgeon General's 2006 report on the subject, "Short exposures to secondhand [tobacco] smoke can cause blood platelets to become stickier, damage the lining of blood vessels, decrease coronary flow velocity reserves, and reduce heart variability, potentially increasing the risk of a heart attack". The American Cancer Society lists "heart disease, lung infections, increased asthma attacks, middle ear infections, and low birth weight" as ramifications of smoker's emission.
Cigarette smoke is a major modifiable risk factor for lung disease, heart disease, and many cancers. Smoke can also be a component of ambient air pollution due to the burning of coal in power plants, forest fires or other sources, although the concentration of pollutants in ambient air is typically much less than that in cigarette smoke. One day of exposure to PM2.5 at a concentration of 880 μg/m3, such as occurs in Beijing, China, is the equivalent of smoking one or two cigarettes in terms of particulate inhalation by weight. The analysis is complicated, however, by the fact that the organic compounds present in various ambient particulates may have a higher carcinogenicity than the compounds in cigarette smoke particulates. Secondhand tobacco smoke is the combination of both sidestream and mainstream smoke emissions from a burning tobacco product. These emissions contain more than 50 carcinogenic chemicals. According to the United States Surgeon General of the United States, Surgeon General's 2006 report on the subject, "Short exposures to secondhand [tobacco] smoke can cause blood platelets to become stickier, damage the lining of blood vessels, decrease coronary flow velocity reserves, and reduce heart variability, potentially increasing the risk of a heart attack". The American Cancer Society lists "heart disease, lung infections, increased asthma attacks, middle ear infections, and low birth weight" as ramifications of smoker's emission.

 Smoke can obscure visibility, impeding occupant exiting from fire areas. In fact, the poor visibility due to the smoke that was in the Worcester Cold Storage Warehouse fire in Worcester, Massachusetts was the reason why the trapped rescue firefighters could not evacuate the building in time. Because of the striking similarity that each floor shared, the dense smoke caused the firefighters to become disoriented.
Smoke can obscure visibility, impeding occupant exiting from fire areas. In fact, the poor visibility due to the smoke that was in the Worcester Cold Storage Warehouse fire in Worcester, Massachusetts was the reason why the trapped rescue firefighters could not evacuate the building in time. Because of the striking similarity that each floor shared, the dense smoke caused the firefighters to become disoriented.
Burning Issues wood smoke SiteShedding new light on wood smoke
https://www.infographicview.com/things-you-need-to-know-about-smoke.html/ 7 things you need to know about smoke detectors] {{Authority control Smoke, Fire





 Smoke is a suspension of airborne
Smoke is a suspension of airborne particulates
Particulates – also known as atmospheric aerosol particles, atmospheric particulate matter, particulate matter (PM) or suspended particulate matter (SPM) – are microscopic particles of solid or liquid matter suspended in the air. The ter ...
and gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
es emitted when a material undergoes combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
or pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''py ...
, together with the quantity of air that is entrained or otherwise mixed into the mass. It is commonly an unwanted by-product
A by-product or byproduct is a secondary product derived from a production process, manufacturing process or chemical reaction; it is not the primary product or service being produced.
A by-product can be useful and marketable or it can be consid ...
of fires (including stoves, candle
A candle is an ignitable wick embedded in wax, or another flammable solid substance such as tallow, that provides light, and in some cases, a fragrance. A candle can also provide heat or a method of keeping time.
A person who makes candles i ...
s, internal combustion engine
An internal combustion engine (ICE or IC engine) is a heat engine in which the combustion of a fuel occurs with an oxidizer (usually air) in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combus ...
s, oil lamps, and fireplace
A fireplace or hearth is a structure made of brick, stone or metal designed to contain a fire. Fireplaces are used for the relaxing ambiance they create and for heating a room. Modern fireplaces vary in heat efficiency, depending on the design.
...
s), but may also be used for pest control (fumigation
Fumigation is a method of pest control or the removal of harmful micro-organisms by completely filling an area with gaseous pesticides—or fumigants—to suffocate or poison the pests within. It is used to control pests in buildings (s ...
), communication (smoke signal
The smoke signal is one of the oldest forms of long-distance communication. It is a form of visual communication used over a long distance. In general smoke signals are used to transmit news, signal danger, or to gather people to a common area ...
s), defensive and offensive capabilities in the military ( smoke screen), cooking
Cooking, cookery, or culinary arts is the art, science and craft of using heat to Outline of food preparation, prepare food for consumption. Cooking techniques and ingredients vary widely, from grilling food over an open fire to using electric ...
, or smoking
Smoking is a practice in which a substance is burned and the resulting smoke is typically breathed in to be tasted and absorbed into the bloodstream. Most commonly, the substance used is the dried leaves of the tobacco plant, which have bee ...
(tobacco
Tobacco is the common name of several plants in the genus '' Nicotiana'' of the family Solanaceae, and the general term for any product prepared from the cured leaves of these plants. More than 70 species of tobacco are known, but the ...
, cannabis
''Cannabis'' () is a genus of flowering plants in the family Cannabaceae. The number of species within the genus is disputed. Three species may be recognized: ''Cannabis sativa'', '' C. indica'', and '' C. ruderalis''. Alternatively ...
, etc.). It is used in rituals where incense
Incense is aromatic biotic material that releases fragrant smoke when burnt. The term is used for either the material or the aroma. Incense is used for aesthetic reasons, religious worship, aromatherapy, meditation, and ceremony. It may also be ...
, sage
Sage or SAGE may refer to:
Plants
* ''Salvia officinalis'', common sage, a small evergreen subshrub used as a culinary herb
** Lamiaceae, a family of flowering plants commonly known as the mint or deadnettle or sage family
** ''Salvia'', a large ...
, or resin
In polymer chemistry and materials science, resin is a solid or highly viscous substance of plant or synthetic origin that is typically convertible into polymers. Resins are usually mixtures of organic compounds. This article focuses on natu ...
is burned to produce a smell for spiritual or magical purposes. It can also be a flavoring agent and preservative.
 Smoke inhalation is the primary cause of death in victims of indoor
Smoke inhalation is the primary cause of death in victims of indoor fire
Fire is the rapid oxidation of a material (the fuel) in the exothermic chemical process of combustion, releasing heat, light, and various reaction Product (chemistry), products.
At a certain point in the combustion reaction, called the ignition ...
s. The smoke kills by a combination of thermal damage, poison
Poison is a chemical substance that has a detrimental effect to life. The term is used in a wide range of scientific fields and industries, where it is often specifically defined. It may also be applied colloquially or figuratively, with a broa ...
ing and pulmonary irritation caused by carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
, hydrogen cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an ...
and other combustion products.
Smoke is an aerosol
An aerosol is a suspension (chemistry), suspension of fine solid particles or liquid Drop (liquid), droplets in air or another gas. Aerosols can be natural or Human impact on the environment, anthropogenic. Examples of natural aerosols are fog o ...
(or mist
Mist is a phenomenon caused by small droplets of water suspended in the cold air, usually by condensation. Physically, it is an example of a dispersion. It is most commonly seen where water vapor in warm, moist air meets sudden cooling, such a ...
) of solid particles and liquid droplets that are close to the ideal range of sizes for Mie scattering
The Mie solution to Maxwell's equations (also known as the Lorenz–Mie solution, the Lorenz–Mie–Debye solution or Mie scattering) describes the scattering of an electromagnetic plane wave by a homogeneous sphere. The solution takes the f ...
of visible light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 te ...
.
Chemical composition
The composition of smoke depends on the nature of the burning fuel and the conditions of combustion. Fires with high availability of oxygen burn at a high temperature and with a small amount of smoke produced; the particles are mostly composed ofash
Ash or ashes are the solid remnants of fires. Specifically, ''ash'' refers to all non-aqueous, non- gaseous residues that remain after something burns. In analytical chemistry, to analyse the mineral and metal content of chemical samples, ash ...
, or with large temperature differences, of condensed aerosol of water. High temperature also leads to production of nitrogen oxide Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
Charge-neutral
*Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide
*Nitrogen dioxide (), nitrogen(IV) oxide
* Nitrogen trioxide (), or n ...
s. Sulfur content yields sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
, or in case of incomplete combustion, hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
. Carbon and hydrogen are almost completely oxidized to carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
and water. Fires burning with lack of oxygen produce a significantly wider palette of compounds, many of them toxic. Partial oxidation of carbon produces carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
, while nitrogen-containing materials can yield hydrogen cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an ...
, ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
, and nitrogen oxides. Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
gas can be produced instead of water. Contents of halogens such as chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
(e.g. in polyvinyl chloride or brominated flame retardant Brominated flame retardants (BFRs) are organobromine compounds that have an inhibitory effect on combustion chemistry and tend to reduce the flammability of products containing them. The brominated variety of commercialized chemical flame retardants ...
s) may lead to the production of hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride ga ...
, phosgene
Phosgene is the organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. Phosgene is a valued and important industrial building block, espe ...
, dioxin
Dioxin may refer to:
* 1,2-Dioxin or 1,4-Dioxin, two unsaturated heterocyclic 6-membered rings where two carbon atoms have been replaced by oxygen atoms, giving the molecular formula C4H4O2
*Dibenzo-1,4-dioxin, the parent compound also known as ...
, and chloromethane, bromomethane
Bromomethane, commonly known as methyl bromide, is an organobromine compound with formula C H3 Br. This colorless, odorless, nonflammable gas is produced both industrially and biologically. It has a tetrahedral shape and it is a recognized ozon ...
and other halocarbon
Halocarbon compounds are chemicals in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms (fluorine, chlorine, bromine or iodine – ) resulting in the formation of organofluorine compounds, organochlori ...
s. Hydrogen fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock i ...
can be formed from fluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often has distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Fluorocarbons and their derivatives are commerci ...
s, whether fluoropolymer
A fluoropolymer is a fluorocarbon-based polymer with multiple carbon–fluorine bonds. It is characterized by a high resistance to solvents, acids, and bases. The best known fluoropolymer is polytetrafluoroethylene under the brand name "Teflon ...
s subjected to fire or halocarbon fire suppression agent
A fire retardant is a substance that is used to slow down or stop the spread of fire or reduce its intensity. This is commonly accomplished by chemical reactions that reduce the flammability of fuels or delay their combustion. Fire retardants m ...
s. Phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
and antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient time ...
oxides and their reaction products can be formed from some fire retardant
A fire retardant is a substance that is used to slow down or stop the spread of fire or reduce its intensity. This is commonly accomplished by chemical reactions that reduce the flammability of fuels or delay their combustion. Fire retardants m ...
additives, increasing smoke toxicity and corrosivity. Pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''py ...
of polychlorinated biphenyl
Polychlorinated biphenyls (PCBs) are highly carcinogenic chemical compounds, formerly used in industrial and consumer products, whose production was banned in the United States by the Toxic Substances Control Act in 1979 and internationally by t ...
s (PCB), e.g. from burning older transformer oil Transformer oil or insulating oil is an oil that is stable at high temperatures and has excellent electrical insulating properties. It is used in oil-filled transformers (wet transformers), some types of high-voltage capacitors, fluorescent lamp b ...
, and to lower degree also of other chlorine-containing materials, can produce 2,3,7,8-tetrachlorodibenzodioxin
2,3,7,8-Tetrachlorodibenzo-''p-''dioxin (TCDD) is a polychlorinated dibenzo''-p-''dioxin (sometimes shortened, though inaccurately, to simply 'dioxin')Tuomisto, Jouko (2019) Dioxins and dioxin-like compounds: toxicity in humans and animals, s ...
, a potent carcinogen
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substan ...
, and other polychlorinated dibenzodioxins
Polychlorinated dibenzodioxins (PCDDs), or simply dioxins, are a group of long-lived polyhalogenated compound, polyhalogenated organic compounds that are primarily anthropogenic, and contribute toxic, Persistent organic pollutant, persistent org ...
. Pyrolysis of fluoropolymers, e.g. teflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chemou ...
, in presence of oxygen yields carbonyl fluoride (which hydrolyzes readily to HF and CO2); other compounds may be formed as well, e.g. carbon tetrafluoride, hexafluoropropylene, and highly toxic perfluoroisobutene
Perfluoroisobutene (PFIB) is the perfluorocarbon counterpart of the hydrocarbon isobutene and has the formula (CF3)2C=CF2. An alkene, it is a colorless gas that is notable as a highly toxic perfluoroalkene. Few simple alkenes are as toxic.
Safet ...
(PFIB).
 Pyrolysis of burning material, especially
Pyrolysis of burning material, especially incomplete combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
or smoldering
Smouldering (British English) or smoldering (American English; see spelling differences) is the slow, flameless form of combustion, sustained by the heat evolved when oxygen directly attacks the surface of a condensed-phase fuel. Many solid mate ...
without adequate oxygen supply, also results in production of a large amount of hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
, both aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or ...
(methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Eart ...
, ethane, ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene i ...
, acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
) and aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
(benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
and its derivates, polycyclic aromatic hydrocarbons; e.g. benzo yrene, studied as a carcinogen, or retene), terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes ar ...
s. It also results in the emission of a range of smaller oxygenated volatile organic compounds (methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
, acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
, hydroxy acetone, methyl acetate and ethyl formate) which are formed as combustion by products as well as less volatile oxygenated organic species such as phenolics, furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly ...
s and furanone
2-Furanone is a heterocyclic organic compound. It is also known as γ-crotonolactone (GCL), as it is formally the lactone derived from γ-hydroxyisocrotonic acid. The chemical is colloquially called "butenolide", and is the parent structure for th ...
s. Heterocyclic compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
s may be also present. Heavier hydrocarbons may condense as tar
Tar is a dark brown or black viscous liquid of hydrocarbons and free carbon, obtained from a wide variety of organic materials through destructive distillation. Tar can be produced from coal, wood, petroleum, or peat. "a dark brown or black bit ...
; smoke with significant tar content is yellow to brown. Combustion of solid fuels can result in the emission of many hundreds to thousands of lower volatility organic compounds in the aerosol phase. Presence of such smoke, soot, and/or brown oily deposits during a fire indicates a possible hazardous situation, as the atmosphere may be saturated with combustible pyrolysis products with concentration above the upper flammability limit, and sudden inrush of air can cause flashover or backdraft.
Presence of sulfur can lead to formation of gases like hydrogen sulfide, carbonyl sulfide, sulfur dioxide, carbon disulfide, and thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
s; especially thiols tend to get adsorbed on surfaces and produce a lingering odor even long after the fire. Partial oxidation of the released hydrocarbons yields in a wide palette of other compounds: aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s (e.g. formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section F ...
, acrolein
Acrolein (systematic name: propenal) is the simplest unsaturated aldehyde. It is a colorless liquid with a piercing, acrid smell. The smell of burnt fat (as when cooking oil is heated to its smoke point) is caused by glycerol in the burning fa ...
, and furfural
Furfural is an organic compound with the formula C4H3OCHO. It is a colorless liquid, although commercial samples are often brown. It has an aldehyde group attached to the 2-position of furan. It is a product of the dehydration of sugars, as occurs ...
), ketones, alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is ...
(often aromatic, e.g. phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it req ...
, guaiacol, syringol, catechol, and cresol
Cresols (also hydroxytoluene or cresylic acid) are a group of aromatic organic compounds. They are widely-occurring phenols (sometimes called ''phenolics'') which may be either natural or manufactured. They are also categorized as methylphenols. ...
s), carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
s (formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Es ...
, acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
, etc.).
The visible particulate matter
Particulates – also known as atmospheric aerosol particles, atmospheric particulate matter, particulate matter (PM) or suspended particulate matter (SPM) – are microscopic particles of solid or liquid matter suspended in the air. The ter ...
in such smokes is most commonly composed of carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
( soot). Other particulates may be composed of drops of condensed tar, or solid particles of ash. The presence of metals in the fuel yields particles of metal oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
s. Particles of inorganic salts may also be formed, e.g. ammonium sulfate, ammonium nitrate, or sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
. Inorganic salts present on the surface of the soot particles may make them hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are no ...
. Many organic compounds, typically the aromatic hydrocarbons, may be also adsorb
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a fl ...
ed on the surface of the solid particles. Metal oxides can be present when metal-containing fuels are burned, e.g. solid rocket
A solid-propellant rocket or solid rocket is a rocket with a rocket engine that uses solid propellants
Rocket propellant is the reaction mass of a rocket. This reaction mass is ejected at the highest achievable velocity from a rocket engine ...
fuels containing aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
. Depleted uranium
Depleted uranium (DU; also referred to in the past as Q-metal, depletalloy or D-38) is uranium with a lower content of the fissile isotope than natural uranium.: "Depleted uranium possesses only 60% of the radioactivity of natural uranium, hav ...
projectiles after impacting the target ignite, producing particles of uranium oxides. Magnetic
Magnetism is the class of physical attributes that are mediated by a magnetic field, which refers to the capacity to induce attractive and repulsive phenomena in other entities. Electric currents and the magnetic moments of elementary particle ...
particles, spherules of magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With the ...
-like ferrous ferric oxide
Iron(II,III) oxide is the chemical compound with formula Fe3O4. It occurs in nature as the mineral magnetite. It is one of a number of iron oxides, the others being iron(II) oxide (FeO), which is rare, and iron(III) oxide (Fe2O3) which also occur ...
, are present in coal smoke; their increase in deposits after 1860 marks the beginning of the Industrial Revolution. (Magnetic iron oxide nanoparticles can be also produced in the smoke from meteorite
A meteorite is a solid piece of debris from an object, such as a comet, asteroid, or meteoroid, that originates in outer space and survives its passage through the atmosphere to reach the surface of a planet or Natural satellite, moon. When the ...
s burning in the atmosphere.) Magnetic remanence
Remanence or remanent magnetization or residual magnetism is the magnetization left behind in a ferromagnetic material (such as iron) after an external magnetic field is removed. Colloquially, when a magnet is "magnetized", it has remanence. The ...
, recorded in the iron oxide particles, indicates the strength of Earth's magnetic field when they were cooled beyond their Curie temperature; this can be used to distinguish magnetic particles of terrestrial and meteoric origin. Fly ash
Fly ash, flue ash, coal ash, or pulverised fuel ash (in the UK) plurale tantum: coal combustion residuals (CCRs)is a coal combustion product that is composed of the particulates (fine particles of burned fuel) that are driven out of coal-fired ...
is composed mainly of silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one ...
and calcium oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, Caustic (substance), caustic, alkaline, crystalline solid at room temperature. The broadly used term "''lime (material), lime''" co ...
. Cenosphere
A cenosphere is a lightweight, inert, hollow sphere made largely of silica and alumina and filled with air or inert gas, typically produced as a coal combustion byproduct at thermal power plants. The color of cenospheres varies from gray to almos ...
s are present in smoke from liquid hydrocarbon fuels. Minute metal particles produced by abrasion can be present in engine smokes. Amorphous silica particles are present in smokes from burning silicone
A silicone or polysiloxane is a polymer made up of siloxane (−R2Si−O−SiR2−, where R = organic group). They are typically colorless oils or rubber-like substances. Silicones are used in sealants, adhesives, lubricants, medicine, cooking ...
s; small proportion of silicon nitride particles can be formed in fires with insufficient oxygen. The silica particles have about 10 nm size, clumped to 70–100 nm aggregates and further agglomerated to chains. Radioactive particles may be present due to traces of uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
, thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high me ...
, or other radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transfer ...
s in the fuel; hot particles can be present in case of fires during nuclear accident
A nuclear and radiation accident is defined by the International Atomic Energy Agency (IAEA) as "an event that has led to significant consequences to people, the environment or the facility. Examples include lethal effects to individuals, lar ...
s (e.g. Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is one of only two nuc ...
) or nuclear war
Nuclear warfare, also known as atomic warfare, is a theoretical military conflict or prepared political strategy that deploys nuclear weaponry. Nuclear weapons are weapons of mass destruction; in contrast to conventional warfare, nuclear w ...
.
Smoke particulates, like other aerosols, are categorized into three modes based on particle size:
* nuclei mode, with geometric mean
In mathematics, the geometric mean is a mean or average which indicates a central tendency of a set of numbers by using the product of their values (as opposed to the arithmetic mean which uses their sum). The geometric mean is defined as the ...
radius between 2.5 and 20 nm, likely forming by condensation of carbon moieties.
* accumulation mode
An aerosol is a suspension (chemistry), suspension of fine solid particles or liquid Drop (liquid), droplets in air or another gas. Aerosols can be natural or Human impact on the environment, anthropogenic. Examples of natural aerosols are fog o ...
, ranging between 75 and 250 nm and formed by coagulation of nuclei mode particles
* coarse mode, with particles in micrometer range
Most of the smoke material is primarily in coarse particles. Those undergo rapid dry precipitation
In the physics of aerosols, deposition is the process by which aerosol particles collect or deposit themselves on solid surfaces, decreasing the concentration of the particles in the air. It can be divided into two sub-processes: ''dry'' and ''w ...
, and the smoke damage in more distant areas outside of the room where the fire occurs is therefore primarily mediated by the smaller particles.
Aerosol of particles beyond visible size is an early indicator of materials in a preignition stage of a fire.
Burning of hydrogen-rich fuel produces water vapor
(99.9839 °C)
, -
, Boiling point
,
, -
, specific gas constant
, 461.5 J/( kg·K)
, -
, Heat of vaporization
, 2.27 MJ/kg
, -
, Heat capacity
, 1.864 kJ/(kg·K)
Water vapor, water vapour or aqueous vapor is the gaseous pha ...
; this results in smoke containing droplets of water. In absence of other color sources (nitrogen oxides, particulates...), such smoke is white and cloud
In meteorology, a cloud is an aerosol consisting of a visible mass of miniature liquid droplets, frozen crystals, or other particles suspended in the atmosphere of a planetary body or similar space. Water or various other chemicals may co ...
-like.
Smoke emissions may contain characteristic trace elements. Vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( pas ...
is present in emissions from oil fired power plants and refineries
A refinery is a production facility composed of a group of chemical engineering unit processes and unit operations refining certain materials or converting raw material into products of value.
Types of refineries
Different types of refineries ar ...
; oil plants also emit some nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
. Coal combustion Fossil fuel power plant#Environmental impacts, produces emissions containing aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
, arsenic, chromium, cobalt, copper, iron, mercury (element), mercury, selenium, and uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
.
Traces of vanadium in high-temperature combustion products form droplets of molten vanadates. These attack the Passivation (chemistry), passivation layers on metals and cause high temperature corrosion, which is a concern especially for internal combustion engine
An internal combustion engine (ICE or IC engine) is a heat engine in which the combustion of a fuel occurs with an oxidizer (usually air) in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combus ...
s. Molten sulfate and lead particulates also have such effect.
Some components of smoke are characteristic of the combustion source. Guaiacol and its derivatives are products of pyrolysis of lignin and are characteristic of wood smoke; other markers are syringol and derivates, and other methoxy phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it req ...
s. Retene, a product of pyrolysis of conifer trees, is an indicator of forest fires. Levoglucosan is a pyrolysis product of cellulose. Hardwood vs softwood smokes differ in the ratio of guaiacols/syringols. Markers for vehicle exhaust include polycyclic aromatic hydrocarbons, hopanes, steranes, and specific nitroarenes (e.g. 1-nitropyrene). The ratio of hopanes and steranes to elemental carbon can be used to distinguish between emissions of gasoline and diesel engines.
Many compounds can be associated with particulates; whether by being adsorption, adsorbed on their surfaces, or by being dissolved in liquid droplets. Hydrogen chloride is well absorbed in the soot particles.
Inert particulate matter can be disturbed and entrained into the smoke. Of particular concern are particles of asbestos.
Deposited hot particles of radioactive fallout and bioaccumulated radioisotopes can be reintroduced into the atmosphere by wildfires and forest fires; this is a concern in e.g. the Zone of alienation containing contaminants from the Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is one of only two nuc ...
.
Polymers are a significant source of smoke. Aromatic side groups, e.g. in polystyrene, enhance generation of smoke. Aromatic groups integrated in the polymer backbone produce less smoke, likely due to significant charring. Aliphatic polymers tend to generate the least smoke, and are non-self-extinguishing. However presence of additives can significantly increase smoke formation. Phosphorus-based and halogen-based flame retardants decrease production of smoke. Higher degree of cross-linking between the polymer chains has such effect too.
Visible and invisible particles of combustion

 The naked eye detects particle sizes greater than 7 µm (micrometres). Visibility, Visible particles emitted from a fire are referred to as smoke. Invisibility, Invisible particles are generally referred to as gas or fumes. This is best illustrated when Toast (food), toasting bread in a toaster. As the bread heats up, the products of combustion increase in size. The fumes initially produced are invisible but become visible if the toast is burnt.
An ionization chamber type smoke detector is technically a product of combustion detector, not a smoke detector. Ionization chamber type smoke detectors detect particles of combustion that are invisible to the naked eye. This explains why they may frequently false alarm from the fumes emitted from the red-hot heating elements of a toaster, before the presence of visible smoke, yet they may fail to activate in the early, low-heat
The naked eye detects particle sizes greater than 7 µm (micrometres). Visibility, Visible particles emitted from a fire are referred to as smoke. Invisibility, Invisible particles are generally referred to as gas or fumes. This is best illustrated when Toast (food), toasting bread in a toaster. As the bread heats up, the products of combustion increase in size. The fumes initially produced are invisible but become visible if the toast is burnt.
An ionization chamber type smoke detector is technically a product of combustion detector, not a smoke detector. Ionization chamber type smoke detectors detect particles of combustion that are invisible to the naked eye. This explains why they may frequently false alarm from the fumes emitted from the red-hot heating elements of a toaster, before the presence of visible smoke, yet they may fail to activate in the early, low-heat smoldering
Smouldering (British English) or smoldering (American English; see spelling differences) is the slow, flameless form of combustion, sustained by the heat evolved when oxygen directly attacks the surface of a condensed-phase fuel. Many solid mate ...
stage of a fire.
Smoke from a typical house fire contains hundreds of different chemicals and fumes. As a result, the damage caused by the smoke can often exceed that caused by the actual heat of the fire. In addition to the physical damage caused by the smoke of a fire
Fire is the rapid oxidation of a material (the fuel) in the exothermic chemical process of combustion, releasing heat, light, and various reaction Product (chemistry), products.
At a certain point in the combustion reaction, called the ignition ...
– which manifests itself in the form of stains – is the often even harder to eliminate problem of a smoky odor. Just as there are contractors that specialize in rebuilding/repairing homes that have been damaged by fire and smoke, fabric restoration companies specialize in restoring fabrics that have been damaged in a fire.
Dangers
Smoke from oxygen-deprived fires contains a significant concentration of compounds that are flammable. A cloud of smoke, in contact with atmospheric oxygen, therefore has the potential of being ignited – either by another open flame in the area, or by its own temperature. This leads to effects like backdraft and flashover. Smoke inhalation is also a danger of smoke that can cause serious injury and death. Many compounds of smoke from fires are highly toxic and/or irritating. The most dangerous iscarbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
leading to carbon monoxide poisoning, sometimes with the additive effects of hydrogen cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an ...
and phosgene
Phosgene is the organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. Phosgene is a valued and important industrial building block, espe ...
. Smoke inhalation can therefore quickly lead to incapacitation and loss of consciousness. Sulfur oxides, hydrogen chloride and hydrogen fluoride in contact with moisture form sulfuric acid, sulfuric, hydrochloric acid, hydrochloric and hydrofluoric acid, which are corrosive to both lungs and materials. When asleep the nose does not sense smoke nor does the brain, but the body will wake up if the lungs become enveloped in smoke and the brain will be stimulated and the person will be awoken. This does not work if the person is incapacitated or under the influence of drugs and/or alcohol.
 Cigarette smoke is a major modifiable risk factor for lung disease, heart disease, and many cancers. Smoke can also be a component of ambient air pollution due to the burning of coal in power plants, forest fires or other sources, although the concentration of pollutants in ambient air is typically much less than that in cigarette smoke. One day of exposure to PM2.5 at a concentration of 880 μg/m3, such as occurs in Beijing, China, is the equivalent of smoking one or two cigarettes in terms of particulate inhalation by weight. The analysis is complicated, however, by the fact that the organic compounds present in various ambient particulates may have a higher carcinogenicity than the compounds in cigarette smoke particulates. Secondhand tobacco smoke is the combination of both sidestream and mainstream smoke emissions from a burning tobacco product. These emissions contain more than 50 carcinogenic chemicals. According to the United States Surgeon General of the United States, Surgeon General's 2006 report on the subject, "Short exposures to secondhand [tobacco] smoke can cause blood platelets to become stickier, damage the lining of blood vessels, decrease coronary flow velocity reserves, and reduce heart variability, potentially increasing the risk of a heart attack". The American Cancer Society lists "heart disease, lung infections, increased asthma attacks, middle ear infections, and low birth weight" as ramifications of smoker's emission.
Cigarette smoke is a major modifiable risk factor for lung disease, heart disease, and many cancers. Smoke can also be a component of ambient air pollution due to the burning of coal in power plants, forest fires or other sources, although the concentration of pollutants in ambient air is typically much less than that in cigarette smoke. One day of exposure to PM2.5 at a concentration of 880 μg/m3, such as occurs in Beijing, China, is the equivalent of smoking one or two cigarettes in terms of particulate inhalation by weight. The analysis is complicated, however, by the fact that the organic compounds present in various ambient particulates may have a higher carcinogenicity than the compounds in cigarette smoke particulates. Secondhand tobacco smoke is the combination of both sidestream and mainstream smoke emissions from a burning tobacco product. These emissions contain more than 50 carcinogenic chemicals. According to the United States Surgeon General of the United States, Surgeon General's 2006 report on the subject, "Short exposures to secondhand [tobacco] smoke can cause blood platelets to become stickier, damage the lining of blood vessels, decrease coronary flow velocity reserves, and reduce heart variability, potentially increasing the risk of a heart attack". The American Cancer Society lists "heart disease, lung infections, increased asthma attacks, middle ear infections, and low birth weight" as ramifications of smoker's emission.
 Smoke can obscure visibility, impeding occupant exiting from fire areas. In fact, the poor visibility due to the smoke that was in the Worcester Cold Storage Warehouse fire in Worcester, Massachusetts was the reason why the trapped rescue firefighters could not evacuate the building in time. Because of the striking similarity that each floor shared, the dense smoke caused the firefighters to become disoriented.
Smoke can obscure visibility, impeding occupant exiting from fire areas. In fact, the poor visibility due to the smoke that was in the Worcester Cold Storage Warehouse fire in Worcester, Massachusetts was the reason why the trapped rescue firefighters could not evacuate the building in time. Because of the striking similarity that each floor shared, the dense smoke caused the firefighters to become disoriented.
Corrosion
Smoke can contain a wide variety of chemicals, many of them aggressive in nature. Examples are hydrochloric acid and hydrobromic acid, produced from halogen-containing plastics andfire retardant
A fire retardant is a substance that is used to slow down or stop the spread of fire or reduce its intensity. This is commonly accomplished by chemical reactions that reduce the flammability of fuels or delay their combustion. Fire retardants m ...
s, hydrofluoric acid released by pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''py ...
of fluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often has distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Fluorocarbons and their derivatives are commerci ...
fire suppression agent
A fire retardant is a substance that is used to slow down or stop the spread of fire or reduce its intensity. This is commonly accomplished by chemical reactions that reduce the flammability of fuels or delay their combustion. Fire retardants m ...
s, sulfuric acid from burning of sulfur-containing materials, nitric acid from high-temperature fires where nitrous oxide gets formed, phosphoric acid and antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient time ...
compounds from P and Sb based fire retardants, and many others. Such corrosion is not significant for structural materials, but delicate structures, especially microelectronics, are strongly affected. Corrosion of circuit board traces, penetration of aggressive chemicals through the casings of parts, and other effects can cause an immediate or gradual deterioration of parameters or even premature (and often delayed, as the corrosion can progress over long time) failure of equipment subjected to smoke. Many smoke components are also electrically conductive; deposition of a conductive layer on the circuits can cause crosstalks and other deteriorations of the operating parameters or even cause short circuits and total failures. Electrical contacts can be affected by corrosion of surfaces, and by deposition of soot and other conductive particles or nonconductive layers on or across the contacts. Deposited particles may adversely affect the performance of optoelectronics by absorbing or scattering the light beams.
Corrosivity of smoke produced by materials is characterized by the corrosion index (CI), defined as material loss rate (angstrom/minute) per amount of material gasified products (grams) per volume of air (m3). It is measured by exposing strips of metal to flow of combustion products in a test tunnel. Polymers containing halogen and hydrogen ( polyvinyl chloride, polyolefins with halogenated additives, etc.) have the highest CI as the corrosive acids are formed directly with water produced by the combustion, polymers containing halogen only (e.g. polytetrafluoroethylene) have lower CI as the formation of acid is limited to reactions with airborne humidity, and halogen-free materials (polyolefins, wood) have the lowest CI. However, some halogen-free materials can also release significant amount of corrosive products.
Smoke damage to electronic equipment can be significantly more extensive than the fire itself. Electrical cable, Cable fires are of special concern; low smoke zero halogen materials are preferable for cable insulation.
When smoke comes into contact with the surface of any substance or structure, the chemicals contained in it are transferred to it. The corrosive properties of the chemicals cause the substance or structure to decompose at a rapid rate. Certain materials or structures absorb these chemicals, which is why clothing, unsealed surfaces, potable water, piping, wood, etc., are replaced in most cases of structural fires.
Health effects of wood smoke
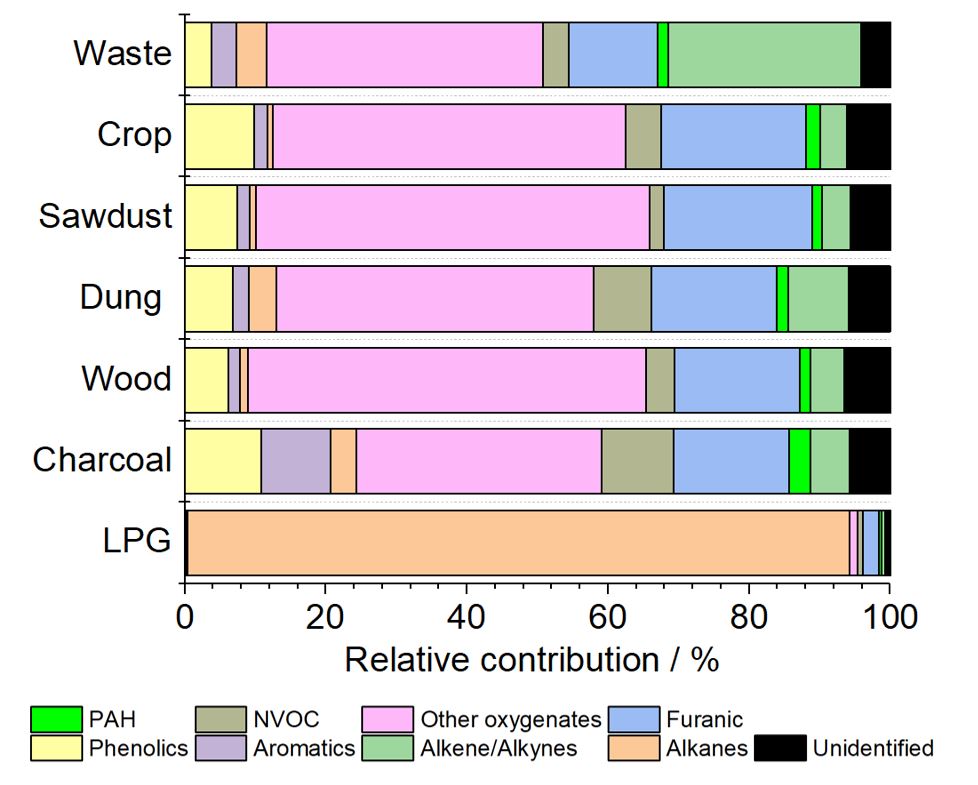
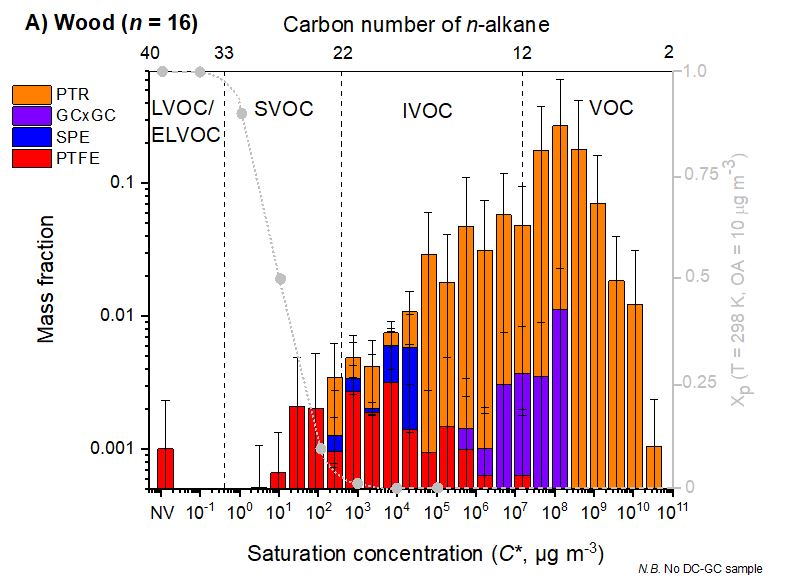
Wood smoke is a major source of air pollution, especially particulate pollution,pollution by polycyclic aromatic hydrocarbons (PAHs) and volatile organic compounds (VOCs) such asformaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section F ...
.
In the United Kingdom domestic combustion, especially for industrial uses, is the largest single source of PM2.5 annually. In some towns and cities in New South Wales, wood smoke may be responsible for 60% of fine particle air pollution in the winter. A year-long sampling campaign in Athens, Greece found a third (31%) of PAH urban air pollution to be caused by wood-burning, roughly as much as that of Diesel locomotive, diesel and Crude oil, oil (33%) and gasoline (29%). It also found that wood-burning is responsible for nearly half (43%) of annual PAH lung cancer-risk compared to the other sources and that wintertime PAH levels were 7 times higher than in other seasons, presumably due to an increased use of Fireplace, fireplaces and heaters. The largest exposure events are periods during the winter with reduced atmospheric dispersion to dilute the accumulated pollution , in particular due to the low wind speeds.
Wood smoke (for example from Wildfire, wildfires) can cause lung damage, artery damage and DNA damage leading to cancer, other respiratory and lung disease and cardiovascular disease. Air pollution, particulate matter and wood smoke may also indirectly cause brain damage because of particulates breaching the cardiovascular system and into the brain, which can increase the risk of developmental disorders, neurodegenerative disorders mental disorders, and suicidal behavior, although studies on the link between Depression (clinical), depression and some air pollutants are not consistent. At least one study has identified "the abundant presence in the human brain of magnetite nanoparticles that match precisely the high-temperature magnetite nanospheres, formed by combustion and/or friction-derived heating, which are prolific in urban, airborne particulate matter (PM)." Air pollution has also been linked to a range of other psychosocial problems.
Measurement
As early as the 15th century Leonardo da Vinci commented at length on the difficulty of assessing smoke, and distinguished between black carbon, black smoke (carbonized particles) and white 'smoke' which is not a smoke at all but merely a suspension of harmless water particulates. Smoke from heating appliances is commonly measured in one of the following ways: In-line capture. A smoke sample is simply sucked through a filter which is weighed before and after the test and the mass of smoke found. This is the simplest and probably the most accurate method, but can only be used where the smoke concentration is slight, as the filter can quickly become blocked. The ASTM smoke pump is a simple and widely used method of in-line capture where a measured volume of smoke is pulled through a filter paper and the dark spot so formed is compared with a standard. Filter/dilution tunnel. A smoke sample is drawn through a tube where it is diluted with air, the resulting smoke/air mixture is then pulled through a filter and weighed. This is the internationally recognized method of measuring smoke fromcombustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combusti ...
.
Electrostatic precipitation. The smoke is passed through an array of metal tubes which contain suspended wires. A (huge) electrical potential is applied across the tubes and wires so that the smoke particles become charged and are attracted to the sides of the tubes. This method can over-read by capturing harmless condensates, or under-read due to the insulating effect of the smoke. However, it is the necessary method for assessing volumes of smoke too great to be forced through a filter, i.e., from bituminous coal.
Ringelmann scale. A measure of smoke color. Invented by Professor Maximilian Ringelmann in Paris in 1888, it is essentially a card with squares of black, white and shades of gray which is held up and the comparative grayness of the smoke judged. Highly dependent on light conditions and the skill of the observer it allocates a grayness number from 0 (white) to 5 (black) which has only a passing relationship to the actual quantity of smoke. Nonetheless, the simplicity of the Ringelmann scale means that it has been adopted as a standard in many countries.
Optical scattering. A light beam is passed through the smoke. A light detector is situated at an angle to the light source, typically at 90°, so that it receives only light reflected from passing particles. A measurement is made of the light received which will be higher as the concentration of smoke particles becomes higher.
Optical obscuration. A light beam is passed through the smoke and a detector opposite measures the light. The more smoke particles are present between the two, the less light will be measured.
Combined optical methods. There are various proprietary optical smoke measurement devices such as the 'nephelometer' or the 'aethalometer' which use several different optical methods, including more than one wavelength of light, inside a single instrument and apply an algorithm to give a good estimate of smoke. It has been claimed that these devices can differentiate types of smoke and so their probable source can be inferred, though this is disputed.
Inference from carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
. Smoke is incompletely burned fuel, carbon monoxide is incompletely burned carbon, therefore it has long been assumed that measurement of CO in flue gas (a cheap, simple and very accurate procedure) will provide a good indication of the levels of smoke. Indeed, several jurisdictions use CO measurement as the basis of smoke control. However it is far from clear how accurate the correspondence is.
Medicinal smoking
Throughout recorded history, humans have used the smoke of medicinal plants to cure illness. A sculpture from Persepolis shows Darius the Great (522–486 BC), the king of Persia, with two censers in front of him for burning Peganum harmala and/or sandalwood Santalum album, which was believed to protect the king from evil and disease. More than 300 plant species in 5 continents are used in smoke form for different diseases. As a method of drug administration, smoking is important as it is a simple, inexpensive, but very effective method of extracting particles containing active agents. More importantly, generating smoke reduces the particle size to a microscopic scale thereby increasing the absorption of its active chemical principles.References
Sources
*External links
Burning Issues wood smoke Site
https://www.infographicview.com/things-you-need-to-know-about-smoke.html/ 7 things you need to know about smoke detectors] {{Authority control Smoke, Fire