Polyfluorene on:
[Wikipedia]
[Google]
[Amazon]
Polyfluorene is a
 Polyfluorenes are an important class of polymers which have the potential to act as both electroactive and
Polyfluorenes are an important class of polymers which have the potential to act as both electroactive and

 The above polymer structure pictured has excellent photoluminescent
The above polymer structure pictured has excellent photoluminescent
 Polyfluorenes are also used in
Polyfluorenes are also used in
polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
with formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwee ...
, consisting of fluorene units linked in a linear chain — specifically, at carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
atoms 2 and 7 in the standard fluorene numbering. It can also be described as a chain of benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
rings linked in ''para'' positions (a polyparaphenylene) with an extra methylene bridge
In organic chemistry, a methylene bridge, methylene spacer, or methanediyl group is any part of a molecule with formula ; namely, a carbon atom bound to two hydrogen atoms and connected by single bonds to two other distinct atoms in the rest of ...
connecting every pair of rings.
The two benzene rings in each unit make polyfluorene an aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
hydrocarbon, specifically conjugated polymer, and give it notable optical and electrical properties, such as efficient photoluminescence
Photoluminescence (abbreviated as PL) is light emission from any form of matter after the absorption of photons (electromagnetic radiation). It is one of many forms of luminescence (light emission) and is initiated by photoexcitation (i.e. photon ...
.
When spoken about as a class, polyfluorenes are derivatives of this polymer, obtained by replacing some of the hydrogen atoms by other chemical group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
s, and/or by substituting other monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
Mo ...
s for some fluorene units. These polymers are being investigated for possible use in light-emitting diode
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (cor ...
s, field-effect transistor
The field-effect transistor (FET) is a type of transistor that uses an electric field to control the flow of current in a semiconductor. FETs (JFETs or MOSFETs) are devices with three terminals: ''source'', ''gate'', and ''drain''. FETs contro ...
s, plastic solar cell
A solar cell, or photovoltaic cell, is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon.
s, and other organic electronic applications. They stand out among other luminescent conjugated polymers because the wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tro ...
of their light output can be tuned through the entire visible spectrum
The visible spectrum is the portion of the electromagnetic spectrum that is visual perception, visible to the human eye. Electromagnetic radiation in this range of wavelengths is called ''visible light'' or simply light. A typical human eye wil ...
by appropriate choice of the substituents
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side ...
.
History
Fluorene, the repeat unit in polyfluorene derivatives, was isolated fromcoal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasi ...
and discovered by Marcellin Berthelot
Pierre Eugène Marcellin Berthelot (; 25 October 1827 – 18 March 1907) was a French chemist and Republican politician noted for the ThomsenBerthelot principle of thermochemistry. He synthesized many organic compounds from inorganic substance ...
prior to 1883.
Its name originates from its interesting fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
(and not to fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
, which is not one of its elements).
Fluorene became the subject of chemical-structure related color variation (visible rather than luminescent), among other things, throughout the early to mid-20th century. Since it was an interesting chromophore
A chromophore is the part of a molecule responsible for its color.
The color that is seen by our eyes is the one not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore is a region in the molec ...
researchers wanted to understand which parts of the molecule were chemically reactive
Reactive may refer to:
*Generally, capable of having a reaction (disambiguation)
*An adjective abbreviation denoting a bowling ball coverstock made of reactive resin
*Reactivity (chemistry)
*Reactive mind
*Reactive programming
See also
*Reactanc ...
, and how substituting these sites influenced the color. For instance, by adding various electron donating or electron accepting moieties to fluorene, and by reacting with bases, researchers were able to change the color of the molecule.
The physical properties of the fluorene molecule were recognizably desirable for polymers; as early as the 1970s researchers began incorporating this moiety into polymers. For instance, because of fluorene’s rigid, planar shape a polymer containing fluorene was shown to exhibit enhanced thermo-mechanical stability.
However, more promising was integrating the optoelectronic properties of fluorene into a polymer. Reports of the oxidative
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
polymerization of fluorene (into a fully conjugated form) exist from at least 1972. However, it was not until after the highly publicized high conductivity of doped polyacetylene, presented in 1977 by Heeger, MacDiarmid and Shirakawa, that substantial interest in the electronic properties of conjugated polymers took off.
As interest in conducting plastics grew, fluorene again found application. The aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
nature of fluorene makes it an excellent candidate component of a conducting polymer because it can stabilize and conduct a charge; in the early 1980s fluorene was electropolymerized into conjugated polymer films with conductivities of 10−4 S cm−1.
The optical properties (such as variable luminescence
Luminescence is spontaneous emission of light by a substance not resulting from heat; or "cold light".
It is thus a form of cold-body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions or stress on a cryst ...
and visible light spectrum absorption) that accompany the extended conjugation in polymers of fluorene have become increasingly attractive for device applications. Throughout the 1990s and into the 2000s, many devices such as organic light-emitting diode
An organic light-emitting diode (OLED or organic LED), also known as organic electroluminescent (organic EL) diode, is a light-emitting diode (LED) in which the emissive electroluminescent layer is a film of organic compound that emits light i ...
s (OLEDs), organic solar cell
An organic solar cell (OSC) or plastic solar cell is a type of photovoltaic that uses organic electronics, a branch of electronics that deals with conductive organic polymers or small organic molecules, for light absorption and charge transport t ...
s., organic thin film transistors, and biosensors
A biosensor is an analytical device, used for the detection of a chemical substance, that combines a biological component with a physicochemical detector.
The ''sensitive biological element'', e.g. tissue, microorganisms, organelles, cell rece ...
have all taken advantage of the luminescent, electronic and absorptive properties of polyfluorenes.
Properties

 Polyfluorenes are an important class of polymers which have the potential to act as both electroactive and
Polyfluorenes are an important class of polymers which have the potential to act as both electroactive and photoactive
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400–7 ...
materials. This in part due to the shape of fluorene. Fluorene is generally planar; p-orbital overlap at the linkage between its two benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
rings results in conjugation
Conjugation or conjugate may refer to:
Linguistics
* Grammatical conjugation, the modification of a verb from its basic form
* Emotive conjugation or Russell's conjugation, the use of loaded language
Mathematics
* Complex conjugation, the chang ...
across the molecule. This in turn allows for a reduced band gap as the excited state molecular orbitals
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of findin ...
are delocalized
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
.
Since the degree of delocalization and the spatial location of the orbitals on the molecule is influenced by the electron donating (or withdrawing) character of its substituents, the band gap
In solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference (in ...
energy can be varied. This chemical control over the band gap directly influences the color of the molecule by limiting the energies of light which it absorbs.
Interest in polyfluorene derivatives has increased because of their high photoluminescence quantum efficiency, high thermal stability, and their facile color tunability, obtained by introducing low-band-gap co-monomers. Research in this field has increased significantly due to its potential application in tuning organic light-emitting diode
An organic light-emitting diode (OLED or organic LED), also known as organic electroluminescent (organic EL) diode, is a light-emitting diode (LED) in which the emissive electroluminescent layer is a film of organic compound that emits light i ...
s (OLEDs). In OLEDs, polyfluorenes are desirable because they are the only family of conjugated polymers that can emit colors spanning the entire visible range with high efficiency and low operating voltage. Furthermore, polyfluorenes are relatively soluble in most solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s, making them ideal for general applications.
Another important quality of polyfluorenes is their thermotropic A liquid crystal phase is thermotropic if its order parameter is determined by temperature. At high temperatures, liquid crystals become an isotropic liquid and at low temperatures, they tend to glassify. In a thermotropic crystal, those phase tr ...
liquid crystal
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal may flow like a liquid, but its molecules may be oriented in a crystal-like way. T ...
linity which allows the polymers to align on rubbed polyimide
Polyimide (sometimes abbreviated PI) is a polymer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, e.g ...
layers. Thermotropic liquid crystallinity refers to the polymers' ability to exhibit a phase transition
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of ...
into the liquid crystal phase as the temperature is changed. This is very important to the development of liquid crystal display
A liquid-crystal display (LCD) is a flat panel display, flat-panel display or other Electro-optic modulator, electronically modulated optical device that uses the light-modulating properties of liquid crystals combined with polarizers. Liqui ...
s (LCDs) because the synthesis of liquid crystal displays requires that the liquid-crystal molecules at the two glass surfaces of the cell be aligned parallel to the two polarizer
A polarizer or polariser is an optical filter that lets light waves of a specific polarization pass through while blocking light waves of other polarizations. It can filter a beam of light of undefined or mixed polarization into a beam of well ...
foils.
This can only be done by coating the inner-surfaces of the cell with a thin, transparent
Transparency, transparence or transparent most often refer to:
* Transparency (optics), the physical property of allowing the transmission of light through a material
They may also refer to:
Literal uses
* Transparency (photography), a still, ...
film of polyamide
A polyamide is a polymer with repeating units linked by amide bonds.
Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made through ...
which is then rubbed with a velvet cloth. Microscopic grooves are then generated in the polyamide layer and the liquid crystal in contact with the polyamide, the polyfluorene, can align in the rubbing direction. In addition to LCDs, polyfluorene can also be used to synthesize light-emitting diode
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (cor ...
s (LEDs). Polyfluorene has led to LEDs that can emit polarized light
Polarization (also polarisation) is a property applying to transverse waves that specifies the geometrical orientation of the oscillations. In a transverse wave, the direction of the oscillation is perpendicular to the direction of motion of the ...
with polarization ratios of more than 20 and with brightness of 100 cd m−2. Even though this is very impressive, it is not sufficient for general applications.
Challenges associated with polyfluorenes
Polyfluorenes often show bothexcimer
An excimer (originally short for excited dimer) is a short-lived dimeric or heterodimeric molecule formed from two species, at least one of which has a valence shell completely filled with electrons (for example, noble gases). In this case, form ...
and aggregate formation upon thermal annealing
In metallurgy and materials science, annealing is a heat treatment that alters the physical and sometimes chemical properties of a material to increase its ductility and reduce its hardness, making it more workable. It involves heating a materi ...
or when current is passed through them. Excimer formation involves the generation of dimerized units of the polymer which emit light at lower energies than the polymer itself. This hinders the use of polyfluorenes for most applications, including light-emitting diode
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (cor ...
s (LED). When excimer or aggregate formation occurs this lowers the efficiency
Efficiency is the often measurable ability to avoid wasting materials, energy, efforts, money, and time in doing something or in producing a desired result. In a more general sense, it is the ability to do things well, successfully, and without ...
of the LEDs by decreasing the efficiency of charge carrier recombination. Excimer formation also causes a red shift in the emission spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a atomic electron transition, transition from a high energy state to a lower energy st ...
.
Polyfluorenes can also undergo decomposition. There are two known ways in which decomposition can occur. The first involves the oxidation of the polymer that leads to the formation of an aromatic ketone, quenching the fluorescence. The second decomposition process results in aggregation leading to a red-shifted fluorescence, reduced intensity, exciton migration and relaxation through excimers.
Researchers have attempted to eliminate excimer formation and enhance the efficiency of polyfluorenes by copolymerizing polyfluorene with anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the Economic production, production of the red dye alizarin and other dyes ...
and end-capping polyfluorenes with bulky groups which could sterically hinder excimer formation. Additionally, researchers have tried adding large substituents at the nine position of the fluorene in order to inhibit excimer and aggregate formation. Furthermore, researchers have tried to improve LEDs by synthesizing fluorene-triarylamine copolymers and other multilayer devices that are based on polyfluorenes that can be cross-linked. These have been found to have brighter fluorescence and reasonable efficiencies.
Aggregation has also been combated by varying the chemical structure. For example, when conjugated polymers aggregate, which is natural in the solid state, their emission can be self-quenched, reducing luminescent quantum yields and reducing luminescent device performance. In opposition to this tendency, researchers have used tri-functional monomers to create highly branched polyfluorenes which do not aggregate due to the bulkiness of the substituents. This design strategy has achieved luminescent quantum yields of 42% in the solid state.
This solution reduces the ease of processability of the material because branched polymers have increased chain entanglement and poor solubility.
Another problem commonly encountered by polyfluorenes is an observed broad green, parasitic emission which detracts from the color purity and efficiency needed for an OLED.
Initially attributed to excimer emission, this green emission has been shown to be due to the formation of ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
defects along the fluorene polymer backbone (oxidation of the nine position on the monomer) when there are incomplete substitution at the nine positions of the fluorene monomer. Routes to combat this involve ensuring full substitution of the monomer’s active site, or including aromatic substituents. These solutions may present structures that lack optimal bulkiness or may be synthetically difficult.
Synthesis and design
Conjugated polymers, such as polyfluorene, can be designed and synthesized with different properties for a wide variety of applications. The color of the molecules can be designed through synthetic control over the electron donating or withdrawing character of the substituents on fluorene or the comonomers in polyfluorene.
Solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
of the polymers are important because solution state processing is very common. Since conjugated polymers, with their planar structure, tend to aggregate, bulky side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a l ...
s are added (to the 9 position of fluorene) to increase the solubility of the polymer.
Oxidative polymerization
The earliest polymerizations of fluorene wereoxidative
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
polymerization with AlCl3 or FeCl3, and more commonly electropolymerization. Electropolymerization is an easy route to obtain thin, insoluble conducting polymer films. However, this technique has a few disadvantages in that it does not provide controlled chain growth polymerizations, and processing and characterization are difficult as a result of its insolubility. Oxidative polymerization produces a similarly poor site-selectivity on the monomer for chain growth resulting in poor control over the regularity of the polymers structure. However, oxidative polymerization does produce soluble polymers (from side-chain containing monomers) which are more easily characterized with nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
.
Cross coupling polymerizations
The design of polymeric properties requires great control over the structure of the polymer. For instance, lowband gap
In solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference (in ...
polymers require regularly alternating electron donating and electron accepting monomers.
More recently, many popular cross-coupling
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = ...
chemistries have been applied to polyfluorenes and have enabled controlled polymerization; Palladium-catalyzed coupling reactions
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = ...
such as Suzuki coupling
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, a ...
, Heck coupling
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a s ...
, etc., as well as nickel catalyzed Yamamoto and Grignard coupling reactions have been applied to polymerization of fluorene derivatives. Such routes have enabled excellent control over the properties of polyfluorenes; the fluorene-thiophene-benzothiadiazole copolymer shown above, with a band gap of 1.78 eV when the side chains are alkoxy
In chemistry, the alkoxy group is an alkyl group which is singularly bonded to oxygen; thus . The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the organic compound ethyl phenyl ether (, also kn ...
, appears blue because it is absorbing in the red wavelengths.

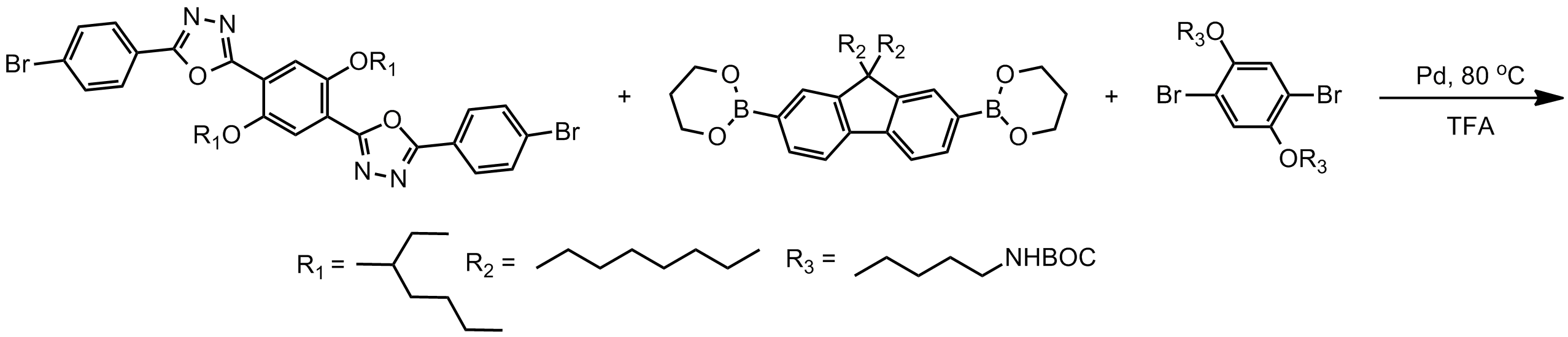
Design
Modern coupling chemistries allow other properties of polyfluorenes to be controlled through implementation of complex molecular designs. The above polymer structure pictured has excellent photoluminescent
The above polymer structure pictured has excellent photoluminescent quantum yield The quantum yield (Φ) of a radiation-induced process is the number of times a specific event occurs per photon absorbed by the system.
Applications
Fluorescence spectroscopy
The fluorescence quantum yield is defined as the ratio of the numb ...
s (partly due to its fluorene monomer) excellent stability (due to its oxadiazole comonomer) good solubility (due to its many and branched alkyl side chains) and has an amine functionalized side chain for ease of tethering to other molecules or to a substrate.
The luminescent color of polyfluorenes can be changed, for example, (from blue to green-yellow) by adding functional groups which participate in excited state intramolecular proton transfer. Exchanging the alkoxy side chains for alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
side groups allows for energy dissipation (and a red-shift in emission) through reversible transfer of a proton from the alcohol to the nitrogen (on the oxadiazole). These complicated molecular structures were engineered to have these properties and were only able to be realized through careful control of their ordering and side group functionality.
Applications
Organic light-emitting diodes (OLEDs)
In recent years many industrial efforts have focused on tuning the color of lights using polyfluorenes. It was found that by doping green or red emitting materials into polyfluorenes one could tune the color emitted by the polymers. Since polyfluorene homopolymers emit higher energy blue light, they can transfer energy via Förster resonance energy transfer (FRET) to lower energy emitters. In addition to doping, color of polyfluorenes can be tuned by copolymerizing the fluorene monomers with other lowband gap
In solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference (in ...
monomers. Researchers at the Dow Chemical Company
The Dow Chemical Company, officially Dow Inc., is an American multinational chemical corporation headquartered in Midland, Michigan, United States. The company is among the three largest chemical producers in the world.
Dow manufactures plastic ...
synthesized several fluorene-based copolymers by alternating copolymerization using 5,5-dibromo-2,2-bithiophene which showed yellow emission and 4,7-dibromo-2,1,3-benzothiadiazole, which showed green emission. Other copolymerizations are also suitable; researchers at IBM performed random copolymerization of fluorene with 3,9(10)-dibromoperylene,4,4-dibromo-R-cyanostilbene, and 1,4-bis(2-(4-bromophenyl)-1-cyanovinyl)-2-(2-ethylhexyl)-5-methoxybenzene. Only a small amount of the co-monomer, approximately 5%, was needed to tune the emission of the polyfluorene from blue to yellow. This example further illustrates that by introducing monomers that have a lower band gap than the fluorene monomer, one can tune the color that is emitted by the polymer.
Substitution at the nine position with various moieties has also been examined as a means to control the color emitted by polyfluorene. In the past researchers have tried putting alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
substituents on the ninth position, however it has been found that by putting bulkier groups, such as alkoxyphenyl groups, the polymers had enhanced blue emission stability and superior polymer light-emitting diode performance (compared to polymers which have alkyl substituents at the ninth position).
Polymer solar cells
 Polyfluorenes are also used in
Polyfluorenes are also used in polymer solar cell
An organic solar cell (OSC) or plastic solar cell is a type of photovoltaic that uses organic electronics, a branch of electronics that deals with conductive organic polymers or small organic molecules, for light absorption and charge transport t ...
s because of their affinity for property tuning. Copolymerization of fluorene with other monomers allows researchers to optimize the absorption and electronic energy levels as a means to increase the photovoltaic performance. For instance, by lowering the band gap of polyfluorenes, the absorption spectrum of the polymer can be adjusted to coincide with the maximum photon flux region of the solar spectrum. This helps the solar cell absorb more of the sun's energy and to increase its energy conversion efficiency
Energy conversion efficiency (''η'') is the ratio between the useful output of an energy conversion machine and the input, in energy terms. The input, as well as the useful output may be chemical, electric power, mechanical work, light (radia ...
; donor-acceptor structured copolymers of fluorene have achieved efficiencies above 4% when their absorption edge was pushed to 700 nm.
The voltage of polymer solar cells has also been increased through the design of polyfluorenes. These devices are typically produced by blending electron accepting and electron donating molecules which help separate charge to produce power. In polymer blend solar cells, the voltage produced by the device is determined by the difference between the electron donating polymer’s highest occupied molecular orbital
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
(HOMO) energy level and the electron accepting molecules lowest unoccupied molecular orbital
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
(LUMO) energy level. By adding electron withdrawing pendant molecules to conjugated polymers, their HOMO energy level can be lowered. For instance by adding electronegative groups on the end of conjugated side chains, researchers lowered the HOMO of a polyfluorene copolymer to −5.30 eV and increased the voltage of a solar cell to 0.99 V.
Typical polymer solar cells utilize fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
molecules as electron acceptors because of their low LUMO energy level (high electron affinity
The electron affinity (''E''ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.
::X(g) + e− → X−(g) + energy
Note that this is ...
). However the tunability of polyfluorenes allows their LUMO to be lowered to a level appropriate for use as an electron acceptor. Thus, polyfluorene copolymers have also been used in polymer:polymer blend solar cells, where their electron accepting, electron conducting and light absorbing properties permit device performance.
References
Further reading
* * * *{{cite thesis , url=http://gradworks.umi.com/31/50/3150130.html , author1=Lee, Shu-Jen , title=Organic Polymer Light-emitting Devices: Optical Modeling, Engineering and Evaluation of Opto-electronic Properties , type=Thesis dissertation , year=2004 , isbn=978-0-496-09054-9 Physical organic chemistry Organic polymers