Pyridine Ring on:
[Wikipedia]
[Google]
[Amazon]
Pyridine is a

 The molecular
The molecular
 Pyridine has a conjugated system of six
Pyridine has a conjugated system of six
 Impure pyridine was undoubtedly prepared by early
Impure pyridine was undoubtedly prepared by early  The contemporary methods of pyridine production had a low yield, and the increasing demand for the new compound urged to search for more efficient routes. A breakthrough came in 1924 when the Russian chemist
The contemporary methods of pyridine production had a low yield, and the increasing demand for the new compound urged to search for more efficient routes. A breakthrough came in 1924 when the Russian chemist
File:4-Bromopyridine.svg, 4-bromopyridine
File:2,2'-Bipyridine.svg, 2,2'- bipyridine
File:Dipicolinic acid.svg, pyridine-2,6-dicarboxylic acid (


 The trimerization of a part of a
The trimerization of a part of a
 The Ciamician–Dennstedt rearrangement entails the ring-expansion of
The Ciamician–Dennstedt rearrangement entails the ring-expansion of  In the Gattermann–Skita synthesis, a malonate ester salt reacts with dichloro
In the Gattermann–Skita synthesis, a malonate ester salt reacts with dichloro Other methods include the
Other methods include the


 Direct nitration of pyridine is sluggish. Pyridine derivatives wherein the nitrogen atom is screened sterically and/or electronically can be obtained by nitration with nitronium tetrafluoroborate (NO2BF4). In this way, 3-nitropyridine can be obtained via the synthesis of 2,6-dibromopyridine followed by nitration and debromination.
Sulfonation of pyridine is even more difficult than nitration. However, pyridine-3-sulfonic acid can be obtained. Reaction with the SO3 group also facilitates addition of sulfur to the nitrogen atom, especially in the presence of a mercury(II) sulfate catalyst.
In contrast to the sluggish nitrations and sulfonations, the
Direct nitration of pyridine is sluggish. Pyridine derivatives wherein the nitrogen atom is screened sterically and/or electronically can be obtained by nitration with nitronium tetrafluoroborate (NO2BF4). In this way, 3-nitropyridine can be obtained via the synthesis of 2,6-dibromopyridine followed by nitration and debromination.
Sulfonation of pyridine is even more difficult than nitration. However, pyridine-3-sulfonic acid can be obtained. Reaction with the SO3 group also facilitates addition of sulfur to the nitrogen atom, especially in the presence of a mercury(II) sulfate catalyst.
In contrast to the sluggish nitrations and sulfonations, the 
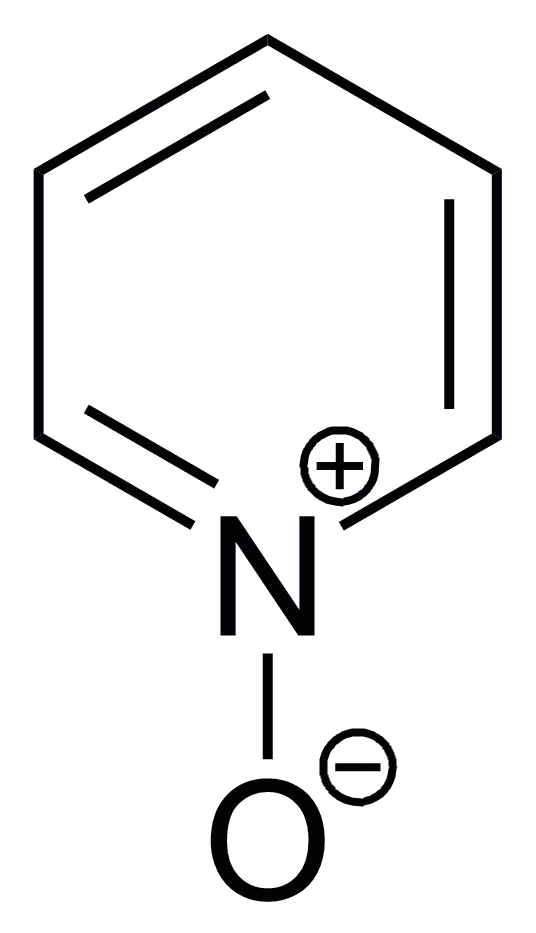 Oxidation of pyridine occurs at nitrogen to give pyridine ''N''-oxide. The oxidation can be achieved with peracids:
:C5H5N + RCO3H → C5H5NO + RCO2H
Some electrophilic substitutions on the pyridine are usefully effected using pyridine ''N''-oxide followed by deoxygenation. Addition of oxygen suppresses further reactions at nitrogen atom and promotes substitution at the 2- and 4-carbons. The oxygen atom can then be removed, e.g. using zinc dust.
Oxidation of pyridine occurs at nitrogen to give pyridine ''N''-oxide. The oxidation can be achieved with peracids:
:C5H5N + RCO3H → C5H5NO + RCO2H
Some electrophilic substitutions on the pyridine are usefully effected using pyridine ''N''-oxide followed by deoxygenation. Addition of oxygen suppresses further reactions at nitrogen atom and promotes substitution at the 2- and 4-carbons. The oxygen atom can then be removed, e.g. using zinc dust.


 Many nucleophilic substitutions occur more easily not with bare pyridine but with pyridine modified with bromine, chlorine, fluorine, or sulfonic acid fragments that then become a leaving group. So fluorine is the best leaving group for the substitution with
Many nucleophilic substitutions occur more easily not with bare pyridine but with pyridine modified with bromine, chlorine, fluorine, or sulfonic acid fragments that then become a leaving group. So fluorine is the best leaving group for the substitution with

 Piperidine is produced by
Piperidine is produced by

 It is also used in the textile industry to improve network capacity of cotton.
It is also used in the textile industry to improve network capacity of cotton.

 As a base, pyridine can be used as the Karl Fischer reagent, but it is usually replaced by alternatives with a more pleasant odor, such as
As a base, pyridine can be used as the Karl Fischer reagent, but it is usually replaced by alternatives with a more pleasant odor, such as
 Exposure to pyridine would normally lead to its inhalation and absorption in the lungs and gastrointestinal tract, where it either remains unchanged or is
Exposure to pyridine would normally lead to its inhalation and absorption in the lungs and gastrointestinal tract, where it either remains unchanged or is
Synthesis and properties of pyridines
at chemsynthesis.com
Synthesis of pyridines (overview of recent methods)
{{Authority control Amine solvents Foul-smelling chemicals Aromatic bases Simple aromatic rings Functional groups Aromatic solvents
basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ...
heterocyclic organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
. It is structurally related to benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
, with one methine group replaced by a nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
atom. It is a highly flammable, weakly alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
ne, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemical
An agrochemical or agrichemical, a contraction of ''agricultural chemical'', is a chemical product used in industrial agriculture. Agrichemical refers to biocides ( pesticides including insecticides, herbicides, fungicides and nematicides) an ...
s, pharmaceutical
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and re ...
s, and vitamin
A vitamin is an organic molecule (or a set of molecules closely related chemically, i.e. vitamers) that is an Nutrient#Essential nutrients, essential micronutrient that an organism needs in small quantities for the proper functioning of its ...
s. Historically, pyridine was produced from coal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasi ...
. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.
Properties
Physical properties
 The molecular
The molecular electric dipole moment
The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system, that is, a measure of the system's overall polarity. The SI unit for electric dipole moment is the coulomb-meter (C⋅m). The ...
is 2.2 debye
The debye (symbol: D) (; ) is a CGS unit (a non- SI metric unit) of electric dipole momentTwo equal and opposite charges separated by some distance constitute an electric dipole. This dipole possesses an electric dipole moment whose value is give ...
s. Pyridine is diamagnetic and has a diamagnetic susceptibility of −48.7 × 10−6 cm3·mol−1. The standard enthalpy of formation
In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state, wi ...
is 100.2 kJ·mol−1 in the liquid phase Lide, p. 5-28 and 140.4 kJ·mol−1 in the gas phase. At 25 °C pyridine has a viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inte ...
of 0.88 mPa/s and thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
of 0.166 W·m−1·K−1. The enthalpy of vaporization is 35.09 kJ·mol−1 at the boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
and normal pressure. The enthalpy of fusion is 8.28 kJ·mol−1 at the melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends ...
.
The critical parameters of pyridine are pressure 6.70 MPa, temperature 620 K and volume 229 cm3·mol−1. In the temperature range 340–426 °C its vapor pressure ''p'' can be described with the Antoine equation
The Antoine equation is a class of semi-empirical correlations describing the relation between vapor pressure and temperature for pure substances. The Antoine equation is derived from the Clausius–Clapeyron relation. The equation was presented ...
:
where ''T'' is temperature, ''A'' = 4.16272, ''B'' = 1371.358 K and ''C'' = −58.496 K.
Structure
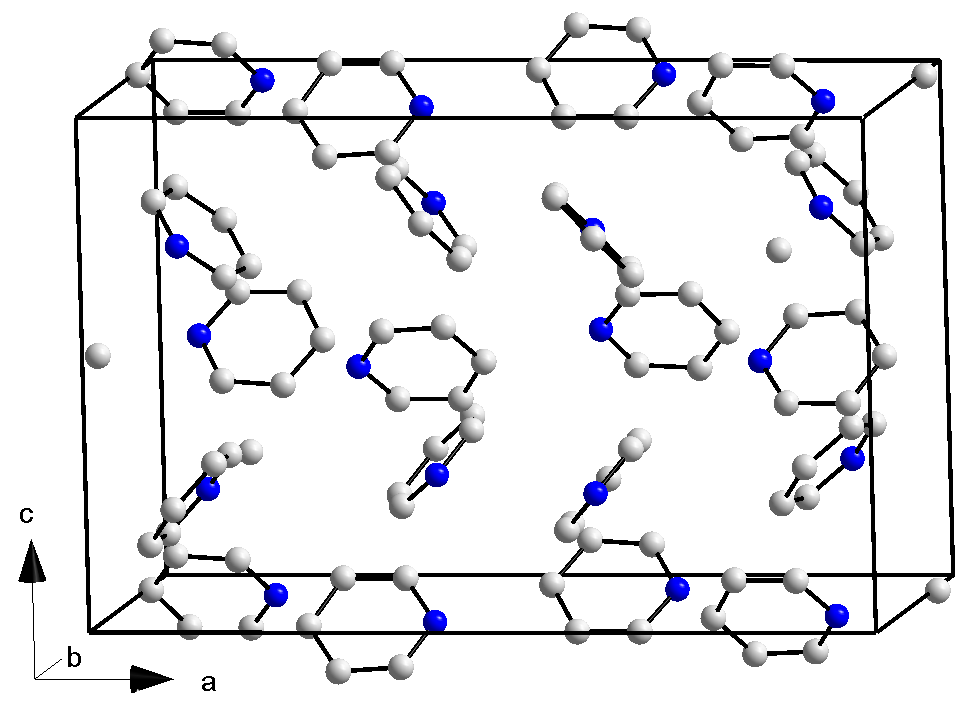
Pyridine ring forms a hexagon. Slight variations of the and distances as well as the bond angles are observed.Crystallography
Pyridine crystallizes in an orthorhombic crystal system with space group ''Pna21'' and lattice parameters ''a'' = 1752 pm, ''b'' = 897 pm, ''c'' = 1135 pm, and 16 formula units per unit cell (measured at 153 K). For comparison, crystallinebenzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
is also orthorhombic, with space group ''Pbca'', ''a'' = 729.2 pm, ''b'' = 947.1 pm, ''c'' = 674.2 pm (at 78 K), but the number of molecules per cell is only 4. This difference is partly related to the lower symmetry of the individual pyridine molecule (C2v vs D6h for benzene). A trihydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understo ...
(pyridine·3H2O) is known; it also crystallizes in an orthorhombic system in the space group ''Pbca'', lattice parameters ''a'' = 1244 pm, ''b'' = 1783 pm, ''c'' = 679 pm and eight formula units per unit cell (measured at 223 K).
Spectroscopy
The optical absorption spectrum of pyridine inhexane
Hexane () is an organic compound, a straight-chain alkane with six carbon atoms and has the molecular formula C6H14.
It is a colorless liquid, odorless when pure, and with boiling points approximately . It is widely used as a cheap, relatively ...
consists of bands at the wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tro ...
s of 195, 251, and 270 nm. With respective extinction coefficients (''ε'') of 7500, 2000, and 450 L·mol−1·cm−1, these bands are assigned to π → π*, π → π*, and n → π* transitions.
The 1H nuclear magnetic resonance (NMR) spectrum shows signals for α-( δ 8.5), γ-(δ7.5) and β-protons (δ7). By contrast, the proton signal for benzene is found at δ7.27. The larger chemical shifts of the α- and γ-protons in comparison to benzene result from the lower electron density in the α- and γ-positions, which can be derived from the resonance structures. The situation is rather similar for the 13C NMR spectra of pyridine and benzene: pyridine shows a triplet at ''δ''(α-C) = 150 ppm, δ(β-C) = 124 ppm and δ(γ-C) = 136 ppm, whereas benzene has a single line at 129 ppm. All shifts are quoted for the solvent-free substances. Pyridine is conventionally detected by the gas chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, ...
and mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is use ...
methods.
Bonding
 Pyridine has a conjugated system of six
Pyridine has a conjugated system of six π electrons
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals ...
that are delocalized over the ring. The molecule is planar and, thus, follows the Hückel criteria for aromatic systems. In contrast to benzene, the electron density is not evenly distributed over the ring, reflecting the negative inductive effect of the nitrogen atom. For this reason, pyridine has a dipole moment and a weaker resonant stabilization than benzene (resonance energy
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
117 kJ·mol−1 in pyridine vs. 150 kJ·mol−1 in benzene).
The ring atoms in the pyridine molecule are sp2-hybridized. The nitrogen is involved in the π-bonding aromatic system using its unhybridized p orbital. The lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
is in an sp2 orbital, projecting outward from the ring in the same plane as the σ bonds. As a result, the lone pair does not contribute to the aromatic system but importantly influences the chemical properties of pyridine, as it easily supports bond formation via an electrophilic attack. However, because of the separation of the lone pair from the aromatic ring system, the nitrogen atom cannot exhibit a positive mesomeric effect.
Many analogues of pyridine are known where N is replaced by other heteroatoms (see figure below). Substitution of one C–H in pyridine with a second N gives rise to the diazine heterocycles (C4H4N2), with the names pyridazine, pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The other ...
, and pyrazine.
History
 Impure pyridine was undoubtedly prepared by early
Impure pyridine was undoubtedly prepared by early alchemists
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim world, ...
by heating animal bones and other organic matter, but the earliest documented reference is attributed to the Scottish scientist Thomas Anderson. In 1849, Anderson examined the contents of the oil obtained through high-temperature heating of animal bones. Among other substances, he separated from the oil a colorless liquid with unpleasant odor, from which he isolated pure pyridine two years later. He described it as highly soluble in water, readily soluble in concentrated acids and salts upon heating, and only slightly soluble in oils.
Owing to its flammability, Anderson named the new substance ''pyridine'', after gr, πῦρ (pyr) meaning ''fire''. The suffix '' idine'' was added in compliance with the chemical nomenclature, as in '' toluidine'', to indicate a cyclic compound containing a nitrogen atom.
The chemical structure of pyridine was determined decades after its discovery. Wilhelm Körner
Wilhelm Körner, later a.k.a. Guglielmo Körner (April 20, 1839 in Cassel – March 29, 1925 in Milan), was a German chemist.
Life
Körner studied chemistry at Giessen, where he graduated in 1860. In 1866, he became assistant to Kekulé at ...
(1869) and James Dewar
Sir James Dewar (20 September 1842 – 27 March 1923) was a British chemist and physicist. He is best known for his invention of the vacuum flask, which he used in conjunction with research into the liquefaction of gases. He also studied ato ...
(1871) suggested that, in analogy between quinoline and naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromati ...
, the structure of pyridine is derived from benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
by substituting one C–H unit with a nitrogen atom. The suggestion by Körner and Dewar was later confirmed in an experiment where pyridine was reduced to piperidine with sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
in ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
. In 1876, William Ramsay combined acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
and hydrogen cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an ...
into pyridine in a red-hot iron-tube furnace. This was the first synthesis of a heteroaromatic compound.
The first major synthesis of pyridine derivatives was described in 1881 by Arthur Rudolf Hantzsch
Arthur Rudolf Hantzsch (7 March 1857 – 14 March 1935) was a German chemist.
Life and work
Hantzsch studied chemistry in Dresden and graduated at the University of Würzburg under Johannes Wislicenus. As a professor, he taught at the Universitie ...
. The Hantzsch pyridine synthesis
The Hantzsch pyridine synthesis or Hantzsch dihydropyridine synthesis is a multi-component organic reaction between an aldehyde such as formaldehyde, 2 equivalents of a β-keto ester such as ethyl acetoacetate and a nitrogen donor such as ammoni ...
typically uses a 2:1:1 mixture of a β- keto acid (often acetoacetate
Acetoacetic acid (also acetoacetate and diacetic acid) is the organic compound with the formula CHCOCHCOOH. It is the simplest beta-keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite stab ...
), an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
(often formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section F ...
), and ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
or its salt as the nitrogen donor. First, a double hydrogenated pyridine is obtained, which is then oxidized to the corresponding pyridine derivative. Emil Knoevenagel
Heinrich Emil Albert Knoevenagel (18 June 1865 – 11 August 1921) was the German chemist who established the Knoevenagel condensation reaction. The Knoevenagel condensation reaction of benzaldehydes with nitroalkanes is a classic general met ...
showed that asymmetrically substituted pyridine derivatives can be produced with this process.
Aleksei Chichibabin
Alekséy Yevgényevich Chichibábin (russian: Алексей Евгеньевич Чичибабин) was a Soviet Union, Soviet/Russian organic chemist, born , Kuzemin village, current Sumy Oblast, Ukraine, died in Paris, France, 15 August 1945. H ...
invented a pyridine synthesis reaction, which was based on inexpensive reagents. This method is still used for the industrial production of pyridine.
Occurrence
Pyridine is not abundant in nature, except for the leaves and roots of belladonna (''Atropa belladonna
''Atropa belladonna'', commonly known as belladonna or deadly nightshade, is a toxic perennial herbaceous plant in the nightshade family Solanaceae, which also includes tomatoes, potatoes, and eggplant (aubergine). It is native to Europe, North ...
'') and in marshmallow ('' Althaea officinalis''). Pyridine derivatives, however, are often part of biomolecules such as alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar ...
s.
In daily life, trace amounts of pyridine are components of the volatile organic compound
Volatile organic compounds (VOCs) are organic compounds that have a high vapour pressure at room temperature. High vapor pressure correlates with a low boiling point, which relates to the number of the sample's molecules in the surrounding air, a ...
s that are produced in roasting and canning
Canning is a method of food preservation in which food is processed and sealed in an airtight container (jars like Mason jars, and steel and tin cans). Canning provides a shelf life that typically ranges from one to five years, although u ...
processes, e.g. in fried chicken, sukiyaki
is a Japanese dish that is prepared and served in the ''nabemono'' (Japanese hot pot) style.
It consists of meat (usually thinly sliced beef) which is slowly cooked or simmered at the table, alongside vegetables and other ingredients, in ...
, roasted coffee, potato chips, and fried bacon
Bacon is a type of salt-cured pork made from various cuts, typically the belly or less fatty parts of the back. It is eaten as a side dish (particularly in breakfasts), used as a central ingredient (e.g., the bacon, lettuce, and tomato sand ...
. Traces of pyridine can be found in Beaufort cheese
Beaufort () is a firm, raw cow's milk cheese associated with the gruyère family. An Alpine cheese, it is produced in Beaufortain, Tarentaise valley and Maurienne, which are located in the Savoie region of the French Alps.
Varieties
There are ...
, vaginal secretion
Vaginal discharge is a mixture of liquid, cells, and bacteria that lubricate and protect the vagina. This mixture is constantly produced by the cells of the vagina and cervix, and it exits the body through the vaginal opening. The composition, amo ...
s, black tea, saliva of those suffering from gingivitis
Gingivitis is a non-destructive disease that causes inflammation of the gums. The most common form of gingivitis, and the most common form of periodontal disease overall, is in response to bacterial biofilms (also called plaque) that is attached ...
, and sunflower honey.
dipicolinic acid
Dipicolinic acid (pyridine-2,6-dicarboxylic acid or PDC and DPA) is a chemical compound which plays a role in the heat resistance of bacterial endospores. It is also used to prepare dipicolinato ligated lanthanide and transition metal complexes f ...
)
File:PyridiniumVerbindungen.svg, General form of the pyridinium cation
Production
Historically, pyridine was extracted fromcoal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasi ...
or obtained as a byproduct of coal gasification
Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (). This is achieved by reacting ...
. The process is labor-consuming and inefficient: coal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasi ...
contains only about 0.1% pyridine, and therefore a multi-stage purification was required, which further reduced the output. Nowadays, most pyridines are synthesized from ammonia, aldehydes, and nitriles, a few combinations of which are suited for pyridine itself. Various name reactions are also known, but they are not practiced on scale.
In 1989, 26,000 tonnes of pyridine was produced worldwide. Other major derivatives are 2-, 3-, 4-methylpyridine
4-Methylpyridine is the organic compound with the formula CH3C5H4N. It is one of the three isomers of methylpyridine. This pungent liquid is a building block for the synthesis of other heterocyclic compounds. Its conjugate acid, the 4-methylpyrid ...
s and 5-ethyl-2-methylpyridine
5-Ethyl-2-methylpyridine is an organic compound with the formula (C2H5)(CH3)C5H3N. One of several isomeric pyridines with this formula, this derivative is of interest because it is efficiently prepared from simple reagents and it is a convenient ...
. The combined scale of these alkylpyridines matches that of pyridine itself. Among the largest 25 production sites for pyridine, eleven are located in Europe (as of 1999). The major producers of pyridine include Evonik Industries, Rütgers Chemicals, Jubilant Life Sciences, Imperial Chemical Industries
Imperial Chemical Industries (ICI) was a British chemical company. It was, for much of its history, the largest manufacturer in Britain.
It was formed by the merger of four leading British chemical companies in 1926.
Its headquarters were at M ...
, and Koei Chemical. Pyridine production significantly increased in the early 2000s, with an annual production capacity of 30,000 tonnes in mainland China alone. The US–Chinese joint venture Vertellus is currently the world leader in pyridine production.
Chichibabin synthesis
TheChichibabin pyridine synthesis
The Chichibabin pyridine synthesis () is a method for synthesizing pyridine rings. The reaction involves the condensation reaction of aldehydes, ketones, α,β-Unsaturated carbonyl compounds, or any combination of the above, with ammonia. It was ...
was reported in 1924 and the basic approach underpins several industrial routes. In its general form, the reaction involves the condensation reaction of aldehydes, ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
, α,β-unsaturated carbonyl compounds, or any combination of the above, in ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
or ammonia derivatives. Application of the Chichibabin pyridine synthesis suffer from low yields, often about 30%, however the precursors are inexpensive. In particular, unsubstituted pyridine is produced from formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section F ...
and acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the mos ...
. First, acrolein
Acrolein (systematic name: propenal) is the simplest unsaturated aldehyde. It is a colorless liquid with a piercing, acrid smell. The smell of burnt fat (as when cooking oil is heated to its smoke point) is caused by glycerol in the burning fa ...
is formed in a Knoevenagel condensation
In organic chemistry, the Knoevenagel condensation () reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation.
A Knoevenagel condensation is a nucleophilic addition of ...
from the acetaldehyde and formaldehyde. The acrolein then condenses with acetaldehyde and ammonia to give dihydropyridine, which is oxidized to pyridine. This process is carried out in a gas phase at 400–450 °C. Typical catalysts are modified forms of alumina and silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one ...
. The reaction has been tailored to produce various methylpyridine Picoline refers to any of three isomers of methylpyridine (CH3C5H4N). They are all colorless liquids with a characteristic smell similar to that of pyridine. They are miscible with water and most organic solvents.
The CAS number of an unspecified ...
s.
Dealkylation and decarboxylation of substituted pyridines
Pyridine can be prepared by dealkylation of alkylated pyridines, which are obtained as byproducts in the syntheses of other pyridines. The oxidative dealkylation is carried out either using air overvanadium(V) oxide
Vanadium(V) oxide (''vanadia'') is the inorganic compound with the formula V2 O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because o ...
catalyst, by vapor-dealkylation on nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
-based catalyst, or hydrodealkylation with a silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
- or platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
-based catalyst. Yields of pyridine up to be 93% can be achieved with the nickel-based catalyst. Pyridine can also be produced by the decarboxylation of nicotinic acid
Niacin, also known as nicotinic acid, is an organic compound and a form of vitamin B3, an essential human nutrient. It can be manufactured by plants and animals from the amino acid tryptophan. Niacin is obtained in the diet from a variet ...
with copper chromite.
Bönnemann cyclization
 The trimerization of a part of a
The trimerization of a part of a nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix ''cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including met ...
molecule and two parts of acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
into pyridine is called Bönnemann cyclization. This modification of the Reppe synthesis
Walter Julius Reppe (29 July 1892 in Göringen – 26 July 1969 in Heidelberg) was a German chemist. He is notable for his contributions to the chemistry of acetylene.
Education and career
Walter Reppe began his study of the natural sciences Un ...
can be activated either by heat or by light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 tera ...
. While the thermal activation requires high pressures and temperatures, the photoinduced cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
proceeds at ambient conditions with CoCp2(cod) (Cp = cyclopentadienyl, cod = 1,5-cyclooctadiene
Cycloocta-1,5-diene is a cyclic hydrocarbon with the chemical formula , specifically .
There are three configurational isomers with this structure, that differ by the arrangement of the four C–C single bonds adjacent to the double bonds. Each ...
) as a catalyst, and can be performed even in water. A series of pyridine derivatives can be produced in this way. When using acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not clas ...
as the nitrile, 2-methylpyridine is obtained, which can be dealkylated to pyridine.
Other methods
The Kröhnke pyridine synthesis provides a fairly general method for generating substituted pyridines using pyridine itself as a reagent which does not become incorporated into the final product. The reaction of pyridine with bromomethyl ketones gives the related pyridinium salt, wherein themethylene group
In organic chemistry, a methylene group is any part of a molecule that consists of two hydrogen atoms bound to a carbon atom, which is connected to the remainder of the molecule by two single bonds. The group may be represented as , where the '< ...
is highly acidic. This species undergoes a Michael-like addition to α,β-unsaturated carbonyls in the presence of ammonium acetate to undergo ring closure and formation of the targeted substituted pyridine as well as pyridinium bromide.
 The Ciamician–Dennstedt rearrangement entails the ring-expansion of
The Ciamician–Dennstedt rearrangement entails the ring-expansion of pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-meth ...
with dichlorocarbene to 3-chloropyridine
3-Chloropyridine is an organohalide with the formula C5H4ClN. It is a colorless liquid that is mainly used as a building block in organic synthesis.
The compound is a substrate for many coupling processes including the Heck reaction, Suzuki react ...
.
 In the Gattermann–Skita synthesis, a malonate ester salt reacts with dichloro
In the Gattermann–Skita synthesis, a malonate ester salt reacts with dichloromethylamine
Methylamine is an organic compound with a formula of . This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine.
Methylamine is sold as a solution in methanol, ...
.
 Other methods include the
Other methods include the Boger pyridine synthesis The Boger pyridine synthesis is a cycloaddition approach to the formation of pyridines named after its inventor Dale L. Boger, who first reported it in 1981. The reaction is a form of inverse-electron demand Diels-Alder reaction in which an enamine ...
and Diels–Alder reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a peric ...
of an alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
and an oxazole
Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak ...
.
Biosynthesis
Several pyridine derivatives play important roles in biological systems. While its biosynthesis is not fully understood,nicotinic acid
Niacin, also known as nicotinic acid, is an organic compound and a form of vitamin B3, an essential human nutrient. It can be manufactured by plants and animals from the amino acid tryptophan. Niacin is obtained in the diet from a variet ...
(vitamin B3) occurs in some bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among ...
, fungi
A fungus ( : fungi or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as a kingdom, separately from ...
, and mammal
Mammals () are a group of vertebrate animals constituting the class Mammalia (), characterized by the presence of mammary glands which in females produce milk for feeding (nursing) their young, a neocortex (a region of the brain), fur or ...
s. Mammals synthesize nicotinic acid through oxidation of the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α- carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic ...
, where an intermediate product, the aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine
In organic chemistry, an aromatic amine is an organic compound consisting of an aroma ...
derivative kynurenine, creates a pyridine derivative, quinolinate
Quinolinic acid (abbreviated QUIN or QA), also known as pyridine-2,3-dicarboxylic acid, is a dicarboxylic acid with a pyridine backbone. It is a colorless solid. It is the biosynthetic precursor to niacin.
Quinolinic acid is a downstream produ ...
and then nicotinic acid. On the contrary, the bacteria ''Mycobacterium tuberculosis
''Mycobacterium tuberculosis'' (M. tb) is a species of pathogenic bacteria in the family Mycobacteriaceae and the causative agent of tuberculosis. First discovered in 1882 by Robert Koch, ''M. tuberculosis'' has an unusual, waxy coating on its c ...
'' and ''Escherichia coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escher ...
'' produce nicotinic acid by condensation of glyceraldehyde 3-phosphate and aspartic acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the pro ...
.
Reactions
Because of the electronegativenitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
in the pyridine ring, therefore pyridine enters less readily into electrophilic aromatic substitution reactions than benzene derivatives. Instead, in terms of its reactivity, pyridine resembles nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor t ...
.
Correspondingly pyridine is more prone to nucleophilic substitution, as evidenced by the ease of metalation Metalation (Alt. spelling: Metallation) is a chemical reaction that forms a bond to a metal. This reaction usually refers to the replacement of a halogen atom in an organic molecule with a metal atom, resulting in an organometallic compound. In the ...
by strong organometallic bases. The reactivity of pyridine can be distinguished for three chemical groups. With electrophiles, electrophilic substitution
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a functional group in a compound, which is typically, but not always, aromatic. Aromatic substitution reactions are characteristic of aromatic compounds ...
takes place where pyridine expresses aromatic properties. With nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
s, pyridine reacts at positions 2 and 4 and thus behaves similar to imines and carbonyls. The reaction with many Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
s results in the addition to the nitrogen atom of pyridine, which is similar to the reactivity of tertiary amines. The ability of pyridine and its derivatives to oxidize, forming amine oxides (''N''-oxides), is also a feature of tertiary amines.
The nitrogen center of pyridine features a basic lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
of electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s. This lone pair does not overlap with the aromatic π-system ring, consequently pyridine is basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ...
, having chemical properties similar to those of tertiary amines. Protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid, ...
gives pyridinium, C5H5NH+.The p''K''a of the conjugate acid
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
(the pyridinium cation) is 5.25. The structures of pyridine and pyridinium are almost identical. The pyridinium cation is isoelectronic with benzene. Pyridinium ''p''- toluenesulfonate (PPTS) is an illustrative pyridinium salt; it is produced by treating pyridine with ''p''-toluenesulfonic acid. In addition to protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid, ...
, pyridine undergoes N-centred alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting ...
, acylation, and ''N''-oxidation. Pyridine and poly(4-vinyl) pyridine have been shown to form conducting molecular wires with remarkable polyenimine structure on UV irradiation, a process which accounts for at least some of the visible light absorption by aged pyridine samples. These wires have been theoretically predicted to be both highly efficient electron donors and acceptors, and yet are resistant to air oxidation.
Electrophilic substitutions
Owing to the decreased electron density in the aromatic system,electrophilic substitution
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a functional group in a compound, which is typically, but not always, aromatic. Aromatic substitution reactions are characteristic of aromatic compounds ...
s are suppressed in pyridine and its derivatives. Friedel–Crafts alkylation or acylation, usually fail for pyridine because they lead only to the addition at the nitrogen atom. Substitutions usually occur at the 3-position, which is the most electron-rich carbon atom in the ring and is, therefore, more susceptible to an electrophilic addition.


 Direct nitration of pyridine is sluggish. Pyridine derivatives wherein the nitrogen atom is screened sterically and/or electronically can be obtained by nitration with nitronium tetrafluoroborate (NO2BF4). In this way, 3-nitropyridine can be obtained via the synthesis of 2,6-dibromopyridine followed by nitration and debromination.
Sulfonation of pyridine is even more difficult than nitration. However, pyridine-3-sulfonic acid can be obtained. Reaction with the SO3 group also facilitates addition of sulfur to the nitrogen atom, especially in the presence of a mercury(II) sulfate catalyst.
In contrast to the sluggish nitrations and sulfonations, the
Direct nitration of pyridine is sluggish. Pyridine derivatives wherein the nitrogen atom is screened sterically and/or electronically can be obtained by nitration with nitronium tetrafluoroborate (NO2BF4). In this way, 3-nitropyridine can be obtained via the synthesis of 2,6-dibromopyridine followed by nitration and debromination.
Sulfonation of pyridine is even more difficult than nitration. However, pyridine-3-sulfonic acid can be obtained. Reaction with the SO3 group also facilitates addition of sulfur to the nitrogen atom, especially in the presence of a mercury(II) sulfate catalyst.
In contrast to the sluggish nitrations and sulfonations, the bromination
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, ...
and chlorination of pyridine proceed well.

Pyridine ''N''-oxide
 Oxidation of pyridine occurs at nitrogen to give pyridine ''N''-oxide. The oxidation can be achieved with peracids:
:C5H5N + RCO3H → C5H5NO + RCO2H
Some electrophilic substitutions on the pyridine are usefully effected using pyridine ''N''-oxide followed by deoxygenation. Addition of oxygen suppresses further reactions at nitrogen atom and promotes substitution at the 2- and 4-carbons. The oxygen atom can then be removed, e.g. using zinc dust.
Oxidation of pyridine occurs at nitrogen to give pyridine ''N''-oxide. The oxidation can be achieved with peracids:
:C5H5N + RCO3H → C5H5NO + RCO2H
Some electrophilic substitutions on the pyridine are usefully effected using pyridine ''N''-oxide followed by deoxygenation. Addition of oxygen suppresses further reactions at nitrogen atom and promotes substitution at the 2- and 4-carbons. The oxygen atom can then be removed, e.g. using zinc dust.
Nucleophilic substitutions
In contrast to benzene ring, pyridine efficiently supports several nucleophilic substitutions. The reason for this is relatively lower electron density of the carbon atoms of the ring. These reactions include substitutions with elimination of ahydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
ion and elimination-additions with formation of an intermediate aryne configuration, and usually proceed at the 2- or 4-position.
organolithium compound
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s. The nucleophilic attack compounds may be alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
s, thiolates, amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s, and ammonia (at elevated pressures).
In general, the hydride ion is a poor leaving group and occurs only in a few heterocyclic reactions. They include the Chichibabin reaction
The Chichibabin reaction (pronounced ' (chē')-chē-bā-bēn) is a method for producing 2-aminopyridine derivatives by the reaction of pyridine with sodium amide. It was reported by Aleksei Chichibabin in 1914. The following is the overall form of ...
, which yields pyridine derivatives aminated at the 2-position. Here, sodium amide is used as the nucleophile yielding 2-aminopyridine. The hydride ion released in this reaction combines with a proton of an available amino group, forming a hydrogen molecule.
Analogous to benzene, nucleophilic substitutions to pyridine can result in the formation of pyridyne
Pyridyne in chemistry is the pyridine analogue of benzyne. Pyridynes are the class of reactive intermediates derived from pyridine. Two isomers exist, the 2,3-pyridine (2,3-didehydropyridine) and the 3,4-pyridyne (3,4-didehydropyridine). The rea ...
intermediates as hetero aryne. For this purpose, pyridine derivatives can be eliminated with good leaving groups using strong bases such as sodium and potassium tert-butoxide. The subsequent addition of a nucleophile to the triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
has low selectivity, and the result is a mixture of the two possible adducts.
Radical reactions
Pyridine supports a series of radical reactions, which is used in itsdimerization
A dimer () (''wikt:di-, di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, Covalent bond, covalent or Intermolecular force, intermolecular. Dimers also have significant im ...
to bipyridines. Radical dimerization of pyridine with elemental sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
or Raney nickel selectively yields 4,4'-bipyridine
4,4′-Bipyridine (abbreviated to 4,4′-bipy or 4,4′-bpy) is an organic compound with the formula . It is one of several isomers of bipyridine. It is a colorless solid that is soluble in organic solvents. is mainly used as a precursor to ''N ...
, or 2,2'-bipyridine
The comma is a punctuation mark that appears in several variants in different languages. It has the same shape as an apostrophe or single closing quotation mark () in many typefaces, but it differs from them in being placed on the baseline o ...
, which are important precursor reagents in the chemical industry. One of the name reactions A name reaction is a chemical reaction named after its discoverers or developers. Among the tens of thousands of organic reactions that are known, hundreds of such reactions are well-known enough to be named after people. Well-known examples include ...
involving free radicals is the Minisci reaction
The Minisci reaction () is a named reaction in organic chemistry. It is a nucleophilic radical substitution to an electron deficient aromatic compound, most commonly the introduction of an alkyl group to a nitrogen containing Heterocyclic compound, ...
. It can produce 2-''tert''-butylpyridine upon reacting pyridine with pivalic acid, silver nitrate
Silver nitrate is an inorganic compound with chemical formula . It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called ''lunar caustic' ...
and ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
in sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
with a yield of 97%.Joule
The joule ( , ; symbol: J) is the unit of energy in the International System of Units (SI). It is equal to the amount of work done when a force of 1 newton displaces a mass through a distance of 1 metre in the direction of the force applied ...
, pp. 125–141
Reactions on the nitrogen atom
Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
s easily add to the nitrogen atom of pyridine, forming pyridinium salts. The reaction with alkyl halides leads to alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting ...
of the nitrogen atom. This creates a positive charge in the ring that increases the reactivity of pyridine to both oxidation and reduction. The Zincke reaction
The Zincke reaction is an organic reaction, named after Theodor Zincke, in which a pyridine is transformed into a pyridinium salt by reaction with 2,4-dinitro-chlorobenzene and a primary amine.
The Zincke reaction should not be confused with th ...
is used for the selective introduction of radicals in pyridinium compounds (it has no relation to the chemical element zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
).
Hydrogenation and reduction
 Piperidine is produced by
Piperidine is produced by hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or S ...
of pyridine with a nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
-, cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, pr ...
-, or ruthenium-based catalyst at elevated temperatures. The hydrogenation of pyridine to piperidine releases 193.8 kJ·mol−1, which is slightly less than the energy of the hydrogenation of benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
(205.3 kJ·mol−1).
Partially hydrogenated derivatives are obtained under milder conditions. For example, reduction with lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li Al H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic ...
yields a mixture of 1,4-dihydropyridine, 1,2-dihydropyridine, and 2,5-dihydropyridine. Selective synthesis of 1,4-dihydropyridine is achieved in the presence of organometallic complexes of magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
and zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
, and (Δ3,4)-tetrahydropyridine is obtained by electrochemical reduction of pyridine.
Lewis basicity and coordination compounds
Pyridine is aLewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
, donating its pair of electrons to a Lewis acid. Its Lewis base properties are discussed in the ECW model. Its relative donor strength toward a series of acids, versus other Lewis bases, can be illustrated by C-B plots. One example is the sulfur trioxide pyridine complex
Sulfur trioxide pyridine complex is the compound with the formula C5H5NSO3. It is a colourless solid that dissolves in polar organic solvents. It is the adduct formed from the Lewis base pyridine and the Lewis acid sulfur trioxide. The compound ...
(melting point 175 °C), which is a sulfation agent used to convert alcohols to sulfate ester
Organosulfates are a class of organic compounds sharing a common functional group with the structure R-O-SO3−. The SO4 core is a sulfate group and the R group is any organic residue. All organosulfates are formally esters derived from alcohols ...
s. Pyridine- borane (, melting point 10–11 °C) is a mild reducing agent.
Transition metal pyridine complexes
Transition metal pyridine complexes encompass many coordination complexes that contain pyridine as a ligand. Most examples are mixed-ligand complexes. Many variants of pyridine are also known to coordinate to metal ions, such as the methylpyridin ...
are numerous.
Typical octahedral complexes have the stoichiometry and . Octahedral homoleptic complexes of the type are rare or tend to dissociate pyridine. Numerous square planar complexes are known, such as Crabtree's catalyst
Crabtree's catalyst is an organoiridium compound with the formula ,5-Cyclooctadiene, C8H12IrTricyclohexylphosphine, P(C6H11)3pyridine, C5H5NF6. It is a homogeneous catalyst for hydrogenation and hydrogen-transfer reactions, developed by Robert ...
. The pyridine ligand replaced during the reaction is restored after its completion.
The ''η''6 coordination mode, as occurs in ''η''6 benzene complexes, is observed only in sterically encumbered derivatives that block the nitrogen center.
Applications
Pesticides and pharmaceuticals
The main use of pyridine is as a precursor to the herbicidesparaquat
Paraquat (trivial name; ), or ''N'',''N''′-dimethyl-4,4′-bipyridinium dichloride (systematic name), also known as methyl viologen, is an organic compound with the chemical formula C6H7N)2l2. It is classified as a viologen, a family of redox ...
and diquat
Diquat is the ISO common name for an organic dication that, as a salt with counterions such as bromide or chloride is used as a contact herbicide that produces desiccation and defoliation. Diquat is no longer approved for use in the European Union ...
. The first synthesis step of insecticide chlorpyrifos consists of the chlorination of pyridine. Pyridine is also the starting compound for the preparation of pyrithione
Pyrithione is the common name of an organosulfur compound with molecular formula , chosen as an abbreviation of pyridinethione, and found in the Persian shallot. It exists as a pair of tautomers, the major form being the thione 1-hydroxy-2(1''H ...
-based fungicide
Fungicides are biocidal chemical compounds or biological organisms used to kill parasitic fungi or their spores. A fungistatic inhibits their growth. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality, ...
s. Cetylpyridinium
Cetylpyridinium chloride (CPC) is a cationic quaternary ammonium compound used in some types of mouthwashes, toothpastes, lozenges, throat sprays, breath sprays, and nasal sprays. It is an antiseptic that kills bacteria and other microorgan ...
and laurylpyridinium, which can be produced from pyridine with a Zincke reaction
The Zincke reaction is an organic reaction, named after Theodor Zincke, in which a pyridine is transformed into a pyridinium salt by reaction with 2,4-dinitro-chlorobenzene and a primary amine.
The Zincke reaction should not be confused with th ...
, are used as antiseptic in oral and dental care products. Pyridine is easily attacked by alkylating agents to give ''N''-alkylpyridinium salts. One example is cetylpyridinium chloride
Cetylpyridinium chloride (CPC) is a cationic quaternary ammonium compound used in some types of mouthwashes, toothpastes, lozenges, throat sprays, breath sprays, and nasal sprays. It is an antiseptic that kills bacteria and other microorganisms ...
.
 It is also used in the textile industry to improve network capacity of cotton.
It is also used in the textile industry to improve network capacity of cotton.
Laboratory use
Pyridine is used as a polar, basic, low-reactive solvent, for example inKnoevenagel condensation
In organic chemistry, the Knoevenagel condensation () reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation.
A Knoevenagel condensation is a nucleophilic addition of ...
s. It is especially suitable for the dehalogenation, where it acts as the base for the elimination reaction. In esterifications and acylations, pyridine activates the carboxylic acid chlorides and anhydrides. Even more active in these reactions are the derivatives 4-dimethylaminopyridine (DMAP) and 4-(1-pyrrolidinyl) pyridine. Pyridine is also used as a base in some condensation reactions.
Reagents
imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole Diazole refers ...
.
Pyridinium chlorochromate
Pyridinium chlorochromate (PCC) is a yellow-orange salt (chemistry), salt with the chemical formula, formula 5H5NH rO3Cl��. It is a reagent in organic synthesis used primarily for organic redox reaction, oxidation of Alcohol (chemistry), al ...
, pyridinium dichromate
The pyridinium dichromate (PDC) or Cornforth reagent is a pyridinium salt of dichromate with the chemical formula 5H5NHsub>2 r2O7 This compound is named after the Australian-British chemist Sir John Warcup Cornforth (b. 1917) who introduced it ...
, and the Collins reagent
Collins reagent is the complex of chromium(VI) oxide with pyridine in dichloromethane. This metal-pyridine complex, a red solid, is used to oxidize primary alcohols to the corresponding aldehydes and secondary alcohols to the corresponding ket ...
(the complex of chromium(VI) oxide
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name.
This compound is a dark-purple s ...
) are used for the oxidation of alcohols.
Hazards
Pyridine is a toxic, flammable liquid with a strong and unpleasant fishy odour. Itsodour threshold
The odor detection threshold is the lowest concentration of a certain odor compound that is perceivable by the human sense of smell. The threshold of a chemical compound is determined in part by its shape, polarity, partial charges, and molecu ...
of 0.04 to 20 ppm is close to its threshold limit of 5 ppm for adverse effects, thus most (but not all) adults will be able to tell when it is present at harmful levels. Pyridine easily dissolves in water and harms both animals and plants in aquatic systems.
Fire
Pyridine has aflash point
The flash point of a material is the "lowest liquid temperature at which, under certain standardized conditions, a liquid gives off vapours in a quantity such as to be capable of forming an ignitable vapour/air mixture". (EN 60079-10-1)
The fl ...
of 17 °C and is therefore highly flammable. Combustion produces toxic fumes which can include bipyridines, nitrogen oxide Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds:
Charge-neutral
*Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide
*Nitrogen dioxide (), nitrogen(IV) oxide
* Nitrogen trioxide (), or n ...
s, and carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
.
Short-term exposure
Pyridine can cause chemical burns on contact with the skin and its fumes may be irritating to the eyes or upon inhalation. Pyridine depresses thenervous system
In biology, the nervous system is the highly complex part of an animal that coordinates its actions and sensory information by transmitting signals to and from different parts of its body. The nervous system detects environmental changes th ...
giving symptoms similar to intoxication with vapor concentrations of above 3600 ppm pose a greater health risk. The effects may have a delayed onset of several hours and include dizziness, headache, lack of coordination, nausea, saliva
Saliva (commonly referred to as spit) is an extracellular fluid produced and secreted by salivary glands in the mouth. In humans, saliva is around 99% water, plus electrolytes, mucus, white blood cells, epithelial cells (from which DNA can be ...
tion, and loss of appetite. They may progress into abdominal pain, pulmonary congestion
Pulmonary edema, also known as pulmonary congestion, is excessive liquid accumulation in the tissue and air spaces (usually alveoli) of the lungs. It leads to impaired gas exchange and may cause hypoxemia and respiratory failure. It is due t ...
and unconsciousness. The lowest known lethal dose (LDLo) for the ingestion of pyridine in humans is 500 mg·kg−1.
Long-term exposure
Prolonged exposure to pyridine may result in liver, heart and kidney damage. Evaluations as a possiblecarcinogenic
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substan ...
agent showed that there is inadequate evidence in humans for the carcinogenicity of pyridine, although there is sufficient evidence in experimental animals. Therefore, IARC IARC may refer to:
* International Aerial Robotics Competition
* International Age Rating Coalition
* International Agency for Research on Cancer
* International Arctic Research Center
* Israel Amateur Radio Club
* iArc IARC may refer to:
* Internat ...
considers pyridine as possibly carcinogenic to humans (Group 2B).
Occurrence
Trace amounts of up to 16 µg·m−3 have been detected in tobacco smoke. Minor amounts of pyridine are released into environment from some industrial processes such as steel manufacture, processing of oil shale,coal gasification Coal gasification is the process of producing syngas—a mixture consisting primarily of carbon monoxide (CO), hydrogen (H2), carbon dioxide (CO2), methane (CH4), and water vapour (H2O)—from coal and water, air and/or oxygen.
Historically, coal ...
, coking
Coking is the heating of coal in the absence of oxygen to a temperature above 600 °C to drive off the volatile components of the raw coal, leaving a hard, strong, porous material of high carbon content called coke. Coke consists almost ent ...
plants and incinerators
Incineration is a list of solid waste treatment technologies, waste treatment process that involves the combustion of substances contained in waste materials. Industrial plants for waste incineration are commonly referred to as waste-to-ene ...
. The atmosphere at oil shale processing plants can contain pyridine concentrations of up to 13 µg·m−3, and 53 µg·m−3 levels were measured in the groundwater
Groundwater is the water present beneath Earth's surface in rock and soil pore spaces and in the fractures of rock formations. About 30 percent of all readily available freshwater in the world is groundwater. A unit of rock or an unconsolidate ...
in the vicinity of a coal gasification plant. According to a study by the US National Institute for Occupational Safety and Health, about 43,000 Americans work in contact with pyridine.
In foods
Pyridine has historically been added to foods to give them a bitter flavour, although this practise is now banned in the U.S. It may still be added toethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
to make it unsuitable for drinking.
Metabolism
 Exposure to pyridine would normally lead to its inhalation and absorption in the lungs and gastrointestinal tract, where it either remains unchanged or is
Exposure to pyridine would normally lead to its inhalation and absorption in the lungs and gastrointestinal tract, where it either remains unchanged or is metabolized
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cell ...
. The major products of pyridine metabolism are ''N''-methylpyridiniumhydroxide, which are formed by ''N''-methyltransferases (e.g., pyridine ''N''-methyltransferase), as well as pyridine ''N''-oxide, and 2-, 3-, and 4-hydroxypyridine, which are generated by the action of monooxygenase
Monooxygenases are enzymes that incorporate one hydroxyl group (−OH) into substrates in many metabolic pathways. In this reaction, the two atoms of dioxygen are reduced to one hydroxyl group and one H2O molecule by the concomitant oxidation o ...
. In humans, pyridine is metabolized only into ''N''-methylpyridiniumhydroxide.
Environmental fate
Pyridine is readily degraded by bacteria to ammonia and carbon dioxide. The unsubstituted pyridine ring degrades more rapidly than picoline,lutidine Lutidine is the trivial name used to describe the chemical compounds which are dimethyl derivatives of pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one met ...
, chloropyridine, or aminopyridine Aminopyridine may refer to any of several chemical compounds:
* 2-Aminopyridine
* 3-Aminopyridine
3-Aminopyridine is an aminopyridine. It is a colorless solid.
Preparation
3-Aminopyridine is prepared by heating nicotinamide with sodium hypobr ...
s, and a number of pyridine degraders have been shown to overproduce riboflavin
Riboflavin, also known as vitamin B2, is a vitamin found in food and sold as a dietary supplement. It is essential to the formation of two major coenzymes, flavin mononucleotide and flavin adenine dinucleotide. These coenzymes are involved in ...
in the presence of pyridine. Ionizable ''N''-heterocyclic compounds, including pyridine, interact with environmental surfaces (such as soils and sediments) via multiple pH-dependent mechanisms, including partitioning to soil organic matter Soil organic matter (SOM) is the organic matter component of soil, consisting of plant and animal detritus at various stages of decomposition, cells and tissues of soil microbes, and substances that soil microbes synthesize. SOM provides numerous b ...
, cation exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
, and surface complexation. Such adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a f ...
to surfaces reduces bioavailability of pyridines for microbial degraders and other organisms, thus slowing degradation rates and reducing ecotoxicity
Ecotoxicity, the subject of study in the field of ecotoxicology (a portmanteau of ecology and toxicology), refers to the biological, chemical or physical stressors that affect ecosystems. Such stressors could occur in the natural environment at ...
.
Nomenclature
The systematic name of pyridine, within theHantzsch–Widman nomenclature
In organic chemistry, Hantzsch–Widman nomenclature, also called the extended Hantzsch–Widman system (named for Arthur Rudolf Hantzsch and ), is a type of systematic chemical nomenclature used for naming heterocyclic parent hydrides having no ...
recommended by the IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
, is '. However, systematic names for simple compounds are used very rarely; instead, heterocyclic nomenclature follows historically established common names. IUPAC discourages the use of in favor of ''pyridine''. The numbering of the ring atoms in pyridine starts at the nitrogen (see infobox). An allocation of positions by letter of the Greek alphabet
The Greek alphabet has been used to write the Greek language since the late 9th or early 8th century BCE. It is derived from the earlier Phoenician alphabet, and was the earliest known alphabetic script to have distinct letters for vowels as we ...
(α-γ) and the substitution pattern nomenclature common for homoaromatic systems (''ortho'', ''meta'', ''para'') are used sometimes. Here α (''ortho''), β (''meta''), and γ (''para'') refer to the 2, 3, and 4 position, respectively. The systematic name for the pyridine derivatives is ''pyridinyl'', wherein the position of the substituted atom is preceded by a number. However, the historical name ''pyridyl'' is encouraged by the IUPAC and used instead of the systematic name. The cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
ic derivative formed by the addition of an electrophile to the nitrogen atom is called '' pyridinium''.
See also
* 6-membered aromatic rings with one carbon replaced by another group: borabenzene, silabenzene,germabenzene
Germabenzene (C5H6Ge) is the parent representative of a group of chemical compounds containing in their molecular structure a benzene ring with a carbon atom replaced by a germanium atom. Germabenzene itself has been studied theoretically, and syn ...
, stannabenzene
Stannabenzene (C5H6Sn) is the parent representative of a group of organotin compounds that are related to benzene with a carbon atom replaced by a tin atom. Stannabenzene itself has been studied by computational chemistry, but has not been isolat ...
, pyridine, phosphorine
Phosphorine (IUPAC name: phosphinine) is a heavier element analog of pyridine, containing a phosphorus atom instead of an aza- moiety. It is also called phosphabenzene and belongs to the phosphaalkene class. It is a colorless liquid that is m ...
, arsabenzene, stibabenzene
Stibinin, also known as stibabenzene, is an organic chemical compound. Stibinin has the chemical formula . The molecule, stibinin, is a derivative of benzene, with one of the carbon atoms in the 6-membered ring replaced by an antimony (Sb) atom. ...
, bismabenzene
Bismabenzene () is the parent representative of a group of organobismuth compounds that are related to benzene with a carbon atom replaced by a bismuth atom. Bismabenzene itself has been synthesised but not isolated because it is too reactive, te ...
, pyrylium
Pyrylium is a cation (positive ion) with formula , consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aroma ...
, thiopyrylium
Thiopyrylium is a cation with the chemical formula C5H5S+. It is analogous to the pyrylium cation with the oxygen atom replaced by a sulfur atom.
Thiopyrylium salts are less reactive than the analogous pyrylium salts due to the higher polarizabi ...
, selenopyrylium
Selenopyrylium is an aromatic heterocyclic compound consisting of a six-membered ring with five carbon atoms and a positively charged selenium atom.
Naming and numbering
Formerly it was named selenapyrylium. However, this is misleading as "selena ...
, telluropyrylium
Telluropyrylium is an aromatic heterocyclic compound consisting of a six member ring with five carbon atoms, and a positively charged tellurium atom. Derivatives of telluropyrylium are important in research of infrared dyes.
Naming and numbering ...
* 6-membered rings with two nitrogen atoms: diazines
* 6-membered rings with three nitrogen atoms: triazines
* 6-membered rings with four nitrogen atoms: tetrazines
* 6-membered rings with five nitrogen atoms: pentazine
* 6-membered rings with six nitrogen atoms: hexazine
References
Bibliography
* *External links
Synthesis and properties of pyridines
at chemsynthesis.com
{{Authority control Amine solvents Foul-smelling chemicals Aromatic bases Simple aromatic rings Functional groups Aromatic solvents