|
Pyrylium
Pyrylium is a cation (positive ion) with formula , consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions. Salts Pyrylium and its derivatives form stable salts with a variety of anions. Derivatives Many important cations are formally derived from pyrylium by substitution of various functional groups for some or all the hydrogens in the ring. The 2,4,6-triphenylpyrilium, referred to as the Katritzky salt, (after Alan R. Katritzky) is an important example used in many modern examples of metal catalyzed cross-couplings. Chemical properties Like other oxonium ions, pyrylium is unstable in neutral water. However, pyrylium is much less reactive than ordinary oxonium ions beca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" (benzoin res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quinol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alexandru Balaban
Alexandru T. Balaban (born April 2, 1931 in Timișoara) is a Romanian chemist who made significant contributions to the fields of organic chemistry, theoretical chemistry, mathematical chemistry, and chemical graph theory. Early life and education Balaban was born in Timișoara, in the western part of Romania. His parents (Teodor and Florica Balaban) paid a lot of attention to Balaban's education, strongly encouraging his fascination with chemistry. In 1935 his family moved to Bucharest, where Balaban attended elementary school. After World War II, in 1945 they moved to Petroșani, where he finished high school. Alexandru Balaban enrolled the Politehnica University of Bucharest in 1949, where he was awarded a Ph.D. in chemistry on April 2, 1959. The topic of his Ph.D. thesis dealt with the restrictions catalyzed by anhydrous aluminium chloride. Career For more than forty years, Balaban held positions at the Chair of Organic Chemistry of the Politehnica University of Buchare ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Costin Nenițescu
Costin D. Neniţescu in some scientific publication written as ''Nenitzescu'' (; 15 July 1902 – 28 July 1970) was a prominent Romanian chemist, and a professor at the Polytechnic University of Bucharest. He was a member of the Romanian Academy, a corresponding member of the German Academy of Sciences in Berlin, and a member of the Leopoldina Academy of Natural Scientists in Halle-Saale. Education and career After completing in 1920 his secondary studies at Gheorghe Lazăr High School, Neniţescu continued his studies at the Polytechnic Institute in Zürich and Ludwig Maximilian University in Munich, where he was one of the favorite students of Hans Fischer. He studied Friedel–Crafts-like reactions in the series of aliphatic hydrocarbons, the mechanism of the isomerization of cyclobasics, the halogen migration in cycles and chains, reactions induced by carbonium ions, and others. He identified a group of naphthenic acids in Romanian crude oil. He searched for ways of obt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cross-coupling Reaction
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon–carbon bond in the product R-R'. Cross-coupling reaction are a subset of coupling reactions. It is often used in arylations. Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed coupling reactions. Mechanism The mechanism generally involves reductive elimination of the organic substituents R and R' on a metal complex of the type LnMR(R') (where L is some arbitrary spectator ligand). The crucial intermediate LnMR(R') is formed in a two step process from a low valence precursor Ln. The oxidative addition of an organic halide (RX) to LnM gives LnMR(X). Subsequ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
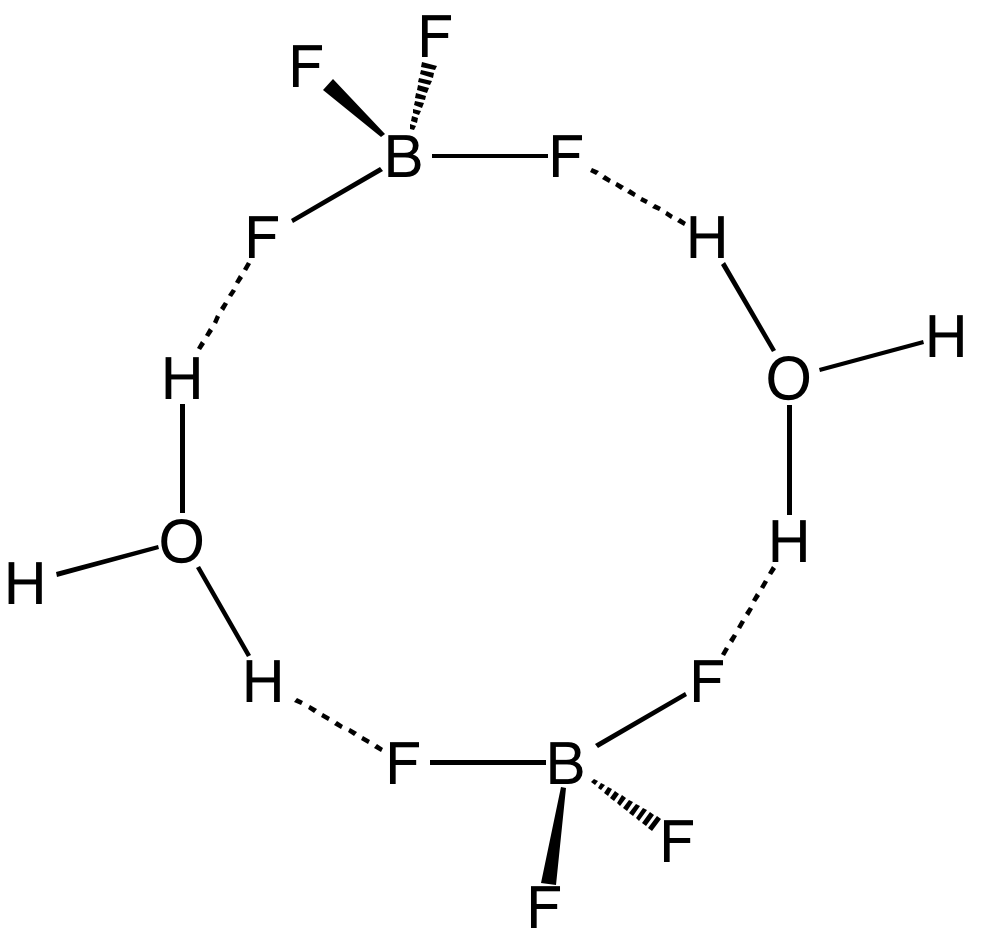
Tetrafluoroboric Acid
Fluoroboric acid or tetrafluoroboric acid (archaically, fluoboric acid) is an inorganic compound with the chemical formula +BF4−], where H+ represents the solvated proton. The solvent can be any suitably Lewis-basic entity. For instance, in water, it can be represented by (oxonium tetrafluoroborate), although more realistically, several water molecules solvate the proton: (H2O)''n''+BF4−]. The ethyl ether solvate is also commercially available: (Et2O)''n''+BF4−], where ''n'' is most likely 2. Unlike other strong acids like H2SO4 or HClO4, the pure unsolvated substance does not exist (see below). It is mainly produced as a precursor to other fluoroborate salts.Gregory K. Friestad, Bruce P. Branchaud "Tetrafluoroboric Acid" E-Eros Encyclopedia of Reagents for Organic Synthesis. It is a strong acid. Fluoroboric acid is corrosive and attacks the skin. It is available commercially as a solution in water and other solvents such as diethyl ether. It is a strong acid with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. It is a colorless liquid with a characteristic almond-like odor. The primary component of bitter almond oil, benzaldehyde can be extracted from a number of other natural sources. Synthetic benzaldehyde is the flavoring agent in imitation almond extract, which is used to flavor cakes and other baked goods. History Benzaldehyde was first extracted in 1803 by the French pharmacist Martrès. His experiments focused on elucidating the nature of amygdalin, the poisonous material found in bitter almonds, the fruit of ''Prunus dulcis''. Further work on the oil by Pierre Robiquet and Antoine Boutron-Charlard, two French chemists, produced benzaldehyde. In 1832, Friedrich Wöhler and Justus von Liebig first synthesized benzaldehyde. Production As of 1999, 7000 tonnes of synthetic and 100 tonnes of natural b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetophenone
Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances. Production Acetophenone is formed as a byproduct of the cumene process, the industrial route for the synthesis of phenol and acetone. In the Hock rearrangement of isopropylbenzene hydroperoxide, migration of a methyl group rather than the phenyl group gives acetophenone and methanol as a result of an alternate rearrangement of the intermediate: :C6H5C(CH3)2O2H -> C6H5C(O)CH3 + CH3OH The cumene process is conducted on such a large scale that even the small amount of acetophenone by-product can be recovered in commercially useful quantities. Acetophenone is also generated from ethylbenzene hydroperoxide. Ethylbenzene hydroperoxide is primarily converted to 1-phenylethanol (α-methylbenzyl alcohol) in the process with a small amount of by-product acetophenone. Acetophenone is recovered or hydrogena ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Condensation Reaction
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a dehydration synthesis. However other molecules can also be lost, such as ammonia, ethanol, acetic acid and hydrogen sulfide. The addition of the two molecules typically proceeds in a step-wise fashion to the addition product, usually in equilibrium, and with loss of a water molecule (hence the name condensation). The reaction may otherwise involve the functional groups of the molecule, and is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and to the biosynthesis of fatty acids. Many variations of condensation reactions exist. Common examples include the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen. Organic chemistry In practice, the term is usually used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular structure. Typical heteroatoms are nitrogen (N), oxygen (O), sulfur (S), phosphorus (P), chlorine (Cl), bromine (Br), and iodine (I), as well as the metals lithium (Li) and magnesium (Mg). Proteins It can also be used with highly specific meanings in specialised contexts. In the description of protein structure, in particular in the Protein Data Bank file format, a heteroatom record (HETATM) describes an atom as belonging to a small molecule cofactor rather than being part of a biopolymer chain. Zeolites In the context of zeolites, the term ''heteroatom'' refers to partial isomorphous substitution of the typical framework atoms (silicon, aluminium, and phosphorus) by other elements such as beryllium, vanadium, and chromium. The goal is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. History The terms ''nucleophile'' and ''electrophile'' were introduced by Christopher Kelk Ingold in 1933, replacing the terms ''anionoid'' and ''cationoid' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to react with air under standard conditions because a passivation layer of nickel oxide forms on the surface that prevents further corrosion. Even so, pure native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere. Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleosynthesis. An iron–nickel mixture is thought to compose Earth's outer and inner cores. Use of nickel (as natural meteoric nickel–iron alloy) has been traced as far back as 3500 BCE. Nickel was first isolated and classified as an e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

2.png)