Benzene on:
[Wikipedia]
[Google]
[Amazon]
Benzene is an organic chemical compound with the
Historic Benzene Formulae Kekulé (original).png, Kekulé's 1872 modification of his 1865 theory, illustrating rapid alternation of double bondsCritics pointed out a problem with Kekulé's original (1865) structure for benzene: Whenever benzene underwent substitution at the ortho position, two distinguishable isomers should have resulted, depending on whether a double bond or a single bond existed between the carbon atoms to which the substituents were attached; however, no such isomers were observed. In 1872, Kekulé suggested that benzene had two complementary structures and that these forms rapidly interconverted, so that if there were a double bond between any pair of carbon atoms at one instant, that double bond would become a single bond at the next instant (and vice versa). To provide a mechanism for the conversion process, Kekulé proposed that the valency of an atom is determined by the frequency with which it collided with its neighbors in a molecule. As the carbon atoms in the benzene ring collided with each other, each carbon atom would collide twice with one neighbor during a given interval and then twice with its other neighbor during the next interval. Thus, a double bond would exist with one neighbor during the first interval and with the other neighbor during the next interval. Therefore, between the carbon atoms of benzene there were no fixed (i.e., constant) and distinct single or double bonds; instead, the bonds between the carbon atoms were identical. Se
pages 86–89
of Auguste Kekulé (1872) "Ueber einige Condensationsprodukte des Aldehyds" (On some condensation products of aldehydes), ''Liebig's Annalen der Chemie und Pharmacie'', 162(1): 77–124, 309–320. From p. 89: ''"Das einfachste Mittel aller Stöße eines Kohlenstoffatoms ergiebt sich aus der Summe der Stöße der beiden ersten Zeiteinheiten, die sich dann periodisch wiederholen. … man sieht daher, daß jedes Kohlenstoffatom mit den beiden anderen, … daß diese Verschiedenheit nur eine scheinbare, aber keine wirkliche ist."'' (The simplest average of all the collisions of a carbon atom n benzenecomes from the sum of the collisions during the first two units of time, which then periodically repeat. … thus one sees that each carbon atom collides equally often with the two others against which it bumps, ndthus stands in exactly the same relation with its two neighbors. The usual structural formula for benzene expresses, of course, only the collisions that occur during ''one'' unit of time, thus during one phase, and so one is led to the view
In 1845, Charles Blachford Mansfield, working under August Wilhelm von Hofmann, isolated benzene from coal tar. Four years later, Mansfield began the first industrial-scale production of benzene, based on the coal-tar method. Gradually, the sense developed among chemists that a number of substances were chemically related to benzene, comprising a diverse chemical family. In 1855, Hofmann used the word " aromatic" to designate this family relationship, after a characteristic property of many of its members. In 1997, benzene was detected in deep space.
 X-ray diffraction shows that all six carbon-carbon bonds in benzene are of the same length, at 140 picometres (pm). The C–C
X-ray diffraction shows that all six carbon-carbon bonds in benzene are of the same length, at 140 picometres (pm). The C–C
C6H5CH3 + H2 -> C6H6 + CH4
This irreversible reaction is accompanied by an equilibrium side reaction that produces
File:Benzene_uses.png, center, Major commodity chemicals and polymers derived from benzene. Clicking on the image loads the appropriate article, 600px, thumb
rect 39 660 435 807 Benzene
rect 665 60 1062 207 Ethylbenzene
rect 665 426 1062 579
 The most widely practiced example of this reaction is the ethylation of benzene.
::
The most widely practiced example of this reaction is the ethylation of benzene.
:: Approximately 24,700,000 tons were produced in 1999. Highly instructive but of far less industrial significance is the Friedel-Crafts alkylation of benzene (and many other aromatic rings) using an
Approximately 24,700,000 tons were produced in 1999. Highly instructive but of far less industrial significance is the Friedel-Crafts alkylation of benzene (and many other aromatic rings) using an 
 Benzene is classified as a carcinogen, which increases the risk of cancer and other illnesses, and is also a notorious cause of bone marrow failure. Substantial quantities of epidemiologic, clinical, and laboratory data link benzene to aplastic anemia, acute leukemia, bone marrow abnormalities and cardiovascular disease. The specific hematologic malignancies that benzene is associated with include: acute myeloid leukemia (AML), aplastic anemia, myelodysplastic syndrome (MDS), acute lymphoblastic leukemia (ALL), and chronic myeloid leukemia (CML).
The
Benzene is classified as a carcinogen, which increases the risk of cancer and other illnesses, and is also a notorious cause of bone marrow failure. Substantial quantities of epidemiologic, clinical, and laboratory data link benzene to aplastic anemia, acute leukemia, bone marrow abnormalities and cardiovascular disease. The specific hematologic malignancies that benzene is associated with include: acute myeloid leukemia (AML), aplastic anemia, myelodysplastic syndrome (MDS), acute lymphoblastic leukemia (ALL), and chronic myeloid leukemia (CML).
The
Benzene
at ''
International Chemical Safety Card 0015
*
Dept. of Health and Human Services: TR-289: Toxicology and Carcinogenesis Studies of Benzene
Video Podcast
of Sir John Cadogan giving a lecture on Benzene since Faraday, in 1991
Substance profile
*
NLM Hazardous Substances Databank – Benzene
{{Authority control Annulenes Aromatic hydrocarbons Aromatic solvents Carcinogens Commodity chemicals GABAA receptor positive allosteric modulators Hazardous air pollutants Hydrocarbon solvents IARC Group 1 carcinogens Immunotoxins Mutagens Occupational safety and health Petrochemicals Simple aromatic rings Six-membered rings Soil contamination Sweet-smelling chemicals Teratogens
molecular formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
C6H6. The benzene molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
is composed of six carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
atoms joined in a planar ring with one hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
.
Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals ...
s between the carbon atoms, benzene is classed as an aromatic hydrocarbon
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past groupin ...
. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline
Gasoline (; ) or petrol (; ) (see ) is a transparent, petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines (also known as petrol engines). It consists mostly of organi ...
. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene
Cumene (isopropylbenzene) is an organic compound that contains a benzene ring with an isopropyl substituent. It is a constituent of crude oil and refined fuels. It is a flammable colorless liquid that has a boiling point of 152 °C. Near ...
, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity.
History
Discovery
The word "''benzene''" derives from "''gum benzoin''" ( benzoin resin), an aromatic resin known to European pharmacists and perfumers since the 16th century as a product of southeast Asia. Anacidic
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a ...
material was derived from benzoin by sublimation, and named "flowers of benzoin", or benzoic acid. The hydrocarbon derived from benzoic acid thus acquired the name benzin, benzol, or benzene. Michael Faraday first isolated and identified benzene in 1825 from the oily residue derived from the production of illuminating gas, giving it the name ''bicarburet of hydrogen''. In 1833, Eilhard Mitscherlich produced it by distilling
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heating ...
benzoic acid (from gum benzoin
Benzoin or benjamin (corrupted pronunciation) is a balsamic resin obtained from the bark of several species of trees in the genus '' Styrax''. It is used in perfumes and some kinds of incense and as a flavoring and medicine (see tincture of ben ...
) and lime
Lime commonly refers to:
* Lime (fruit), a green citrus fruit
* Lime (material), inorganic materials containing calcium, usually calcium oxide or calcium hydroxide
* Lime (color), a color between yellow and green
Lime may also refer to:
Botany ...
. He gave the compound the name ''benzin''. In 1836, the French chemist Auguste Laurent named the substance "phène"; this word has become the root of the English word " phenol", which is hydroxylated benzene, and "phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
", the radical formed by abstraction of a hydrogen atom (free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
H•) from benzene.
pages 86–89
of Auguste Kekulé (1872) "Ueber einige Condensationsprodukte des Aldehyds" (On some condensation products of aldehydes), ''Liebig's Annalen der Chemie und Pharmacie'', 162(1): 77–124, 309–320. From p. 89: ''"Das einfachste Mittel aller Stöße eines Kohlenstoffatoms ergiebt sich aus der Summe der Stöße der beiden ersten Zeiteinheiten, die sich dann periodisch wiederholen. … man sieht daher, daß jedes Kohlenstoffatom mit den beiden anderen, … daß diese Verschiedenheit nur eine scheinbare, aber keine wirkliche ist."'' (The simplest average of all the collisions of a carbon atom n benzenecomes from the sum of the collisions during the first two units of time, which then periodically repeat. … thus one sees that each carbon atom collides equally often with the two others against which it bumps, ndthus stands in exactly the same relation with its two neighbors. The usual structural formula for benzene expresses, of course, only the collisions that occur during ''one'' unit of time, thus during one phase, and so one is led to the view
hat
A hat is a head covering which is worn for various reasons, including protection against weather conditions, ceremonial reasons such as university graduation, religious reasons, safety, or as a fashion accessory. Hats which incorporate mecha ...
doubly substituted derivatives f benzenemust be different at positions 1,2 and 1,6 f the benzene ring If the idea hat wasjust presented—or a similar one—can be regarded as correct, then tfollows therefrom that this difference etween the bonds at positions 1,2 and 1,6is only an apparent ne not a real ne)
Ring formula
Theempirical formula
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the ...
for benzene was long known, but its highly polyunsaturated
In nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such compounds, most commonly those that occur in living beings or in food.
The term often refers specifically to triglycerides (triple es ...
structure, with just one hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
atom for each carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
atom, was challenging to determine. Archibald Scott Couper in 1858 and Johann Josef Loschmidt
Johann Josef Loschmidt (15 March 1821 – 8 July 1895), who referred to himself mostly as Josef Loschmidt (omitting his first name), was a notable Austrian scientist who performed ground-breaking work in chemistry, physics (thermodynamics, optics, ...
in 1861 suggested possible structures that contained multiple double bonds or multiple rings, but too little evidence was then available to help chemists decide on any particular structure.
In 1865, the German chemist Friedrich August Kekulé published a paper in French (for he was then teaching in Francophone Belgium) suggesting that the structure contained a ring of six carbon atoms with alternating single and double bonds. The next year he published a much longer paper in German on the same subject. On p. 100, Kekulé suggests that the carbon atoms of benzene could form a "chaîne fermée" (a closed chain, a loop). Kekulé used evidence that had accumulated in the intervening years—namely, that there always appeared to be only one isomer of any monoderivative of benzene, and that there always appeared to be exactly three isomers of every disubstituted derivative—now understood to correspond to the ortho, meta, and para patterns of arene substitution—to argue in support of his proposed structure. Kekulé's symmetrical ring could explain these curious facts, as well as benzene's 1:1 carbon-hydrogen ratio.
The new understanding of benzene, and hence of all aromatic compounds, proved to be so important for both pure and applied chemistry that in 1890 the German Chemical Society organized an elaborate appreciation in Kekulé's honor, celebrating the twenty-fifth anniversary of his first benzene paper. Here Kekulé spoke of the creation of the theory. He said that he had discovered the ring shape of the benzene molecule after having a reverie or day-dream of a snake biting its own tail (this is a common symbol in many ancient cultures known as the Ouroboros
The ouroboros or uroboros () is an ancient symbol depicting a serpent or dragon eating its own tail. The ouroboros entered Western tradition via ancient Egyptian iconography and the Greek magical tradition. It was adopted as a symbol in Gnost ...
or endless knot
Endless knot in a Burmese Pali manuscript
The endless knot or eternal knot is a symbolic knot and one of the Eight Auspicious Symbols. It is an important symbol in Hinduism, Jainism and Buddhism. It is an important cultural marker in place ...
). This vision, he said, came to him after years of studying the nature of carbon-carbon bonds. This was seven years after he had solved the problem of how carbon atoms could bond to up to four other atoms at the same time. Curiously, a similar, humorous depiction of benzene had appeared in 1886 in a pamphlet entitled ''Berichte der Durstigen Chemischen Gesellschaft'' (Journal of the Thirsty Chemical Society), a parody of the ''Berichte der Deutschen Chemischen Gesellschaft'', only the parody had monkeys seizing each other in a circle, rather than snakes as in Kekulé's anecdote. Some historians have suggested that the parody was a lampoon of the snake anecdote, possibly already well known through oral transmission even if it had not yet appeared in print. Kekulé's 1890 speech in which this anecdote appeared has been translated into English. If the anecdote is the memory of a real event, circumstances mentioned in the story suggest that it must have happened early in 1862.
In 1929, the cyclic nature of benzene was finally confirmed by the crystallographer Kathleen Lonsdale
Dame Kathleen Lonsdale ( Yardley; 28 January 1903 – 1 April 1971) was an Irish-born British pacifist, prison reformer and crystallographer. She proved, in 1929, that the benzene ring is flat by using X-ray diffraction methods to elucidate t ...
using X-ray diffraction methods. Using large crystals of hexamethylbenzene
Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each ...
, a benzene derivative with the same core of six carbon atoms, Lonsdale obtained diffraction patterns. Through calculating more than thirty parameters, Lonsdale demonstrated that the benzene ring could not be anything but a flat hexagon, and provided accurate distances for all carbon-carbon bonds in the molecule.
Nomenclature
The German chemist Wilhelm Körner suggested the prefixes ortho-, meta-, para- to distinguish di-substituted benzene derivatives in 1867; however, he did not use the prefixes to distinguish the relative positions of the substituents on a benzene ring. It was the German chemist Carl Gräbe who, in 1869, first used the prefixes ortho-, meta-, para- to denote specific relative locations of the substituents on a di-substituted aromatic ring (viz, naphthalene). In 1870, the German chemist Viktor Meyer first applied Gräbe's nomenclature to benzene.Early applications
In the 19th and early 20th centuries, benzene was used as an after-shave lotion because of its pleasant smell. Prior to the 1920s, benzene was frequently used as an industrial solvent, especially fordegreasing
Degreasing, often called defatting or fat trimming, is the removal of fatty acids from an object. In culinary science, degreasing is done with the intention of reducing the fat content of a meal.
Degreasing food
Degreasing is often used by diete ...
metal. As its toxicity became obvious, benzene was supplanted by other solvents, especially toluene (methylbenzene), which has similar physical properties but is not as carcinogenic.
In 1903, Ludwig Roselius
Ludwig Roselius (2 June 1874 – 15 May 1943) was a German coffee merchant and founder of the company Kaffee HAG. He was born in Bremen and is credited with the development of commercial decaffeination of coffee. As a patron, he supported arti ...
popularized the use of benzene to decaffeinate coffee
Coffee is a drink prepared from roasted coffee beans. Darkly colored, bitter, and slightly acidic, coffee has a stimulating effect on humans, primarily due to its caffeine content. It is the most popular hot drink in the world.
Seeds of ...
. This discovery led to the production of Sanka. This process was later discontinued. Benzene was historically used as a significant component in many consumer products such as liquid wrench, several paint stripper
Paint stripper, or paint remover, is a chemical product designed to remove paint, wood finishing, finishes, and coatings, while also cleaning the underlying surface.
The product's material safety data sheet provides more safety information than ...
s, rubber cements, spot removers, and other products. Manufacture of some of these benzene-containing formulations ceased in about 1950, although Liquid Wrench continued to contain significant amounts of benzene until the late 1970s.
Occurrence
Trace amounts of benzene are found in petroleum and coal. It is a byproduct of the incomplete combustion of many materials. For commercial use, untilWorld War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the vast majority of the world's countries—including all of the great powers—forming two opposing ...
, much of benzene was obtained as a by-product of coke production (or "coke-oven light oil") for the steel industry. However, in the 1950s, increased demand for benzene, especially from the growing polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s industry, necessitated the production of benzene from petroleum. Today, most benzene comes from the petrochemical industry, with only a small fraction being produced from coal. Benzene molecules have been detected on Mars
Mars is the fourth planet from the Sun and the second-smallest planet in the Solar System, only being larger than Mercury. In the English language, Mars is named for the Roman god of war. Mars is a terrestrial planet with a thin at ...
.
Structure
bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of ...
s are greater than a double bond (135 pm) but shorter than a single bond (147 pm). This intermediate distance is caused by electron delocalization
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
: the electrons for C=C bonding are distributed equally between each of the six carbon atoms. Benzene has 6 hydrogen atoms, fewer than the corresponding parent alkane, hexane, which has 14. Benzene and cyclohexane have a similar structure, only the ring of delocalized electrons and the loss of one hydrogen per carbon distinguishes it from cyclohexane. The molecule is planar. The molecular orbital description involves the formation of three delocalized π orbitals spanning all six carbon atoms, while the valence bond description involves a superposition of resonance structures
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
. It is likely that this stability contributes to the peculiar molecular and chemical properties known as aromaticity. To accurately reflect the nature of the bonding, benzene is often depicted with a circle inside a hexagonal arrangement of carbon atoms.
Derivatives of benzene occur sufficiently often as a component of organic molecules, so much so that the Unicode
Unicode, formally The Unicode Standard,The formal version reference is is an information technology standard for the consistent encoding, representation, and handling of text expressed in most of the world's writing systems. The standard, wh ...
Consortium has allocated a symbol in the Miscellaneous Technical
Miscellaneous Technical is a Unicode block ranging from U+2300 to U+23FF, which contains various common symbols which are related to and used in the various technical, programming language, and academic professions. For example:
* Symbol ⌂ (H ...
block with the code U+232C (⌬) to represent it with three double bonds, and U+23E3 (⏣) for a delocalized version.
Benzene derivatives
Many important chemical compounds are derived from benzene by replacing one or more of its hydrogen atoms with another functional group. Examples of simple benzene derivatives are phenol, toluene, andaniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starti ...
, abbreviated PhOH, PhMe, and PhNH2, respectively. Linking benzene rings gives biphenyl
Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one ...
, C6H5–C6H5. Further loss of hydrogen gives "fused" aromatic hydrocarbons, such as naphthalene, anthracene, phenanthrene, and pyrene. The limit of the fusion process is the hydrogen-free allotrope of carbon, graphite.
In heterocycles, carbon atoms in the benzene ring are replaced with other elements. The most important variations contain nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
. Replacing one CH with N gives the compound pyridine, C5H5N. Although benzene and pyridine are ''structurally'' related, benzene cannot be converted into pyridine. Replacement of a second CH bond with N gives, depending on the location of the second N, pyridazine
Pyridazine is an aromatic, heterocyclic, organic compound with the molecular formula . It contains a six-membered ring with two adjacent nitrogen atoms. It is a colorless liquid with a boiling point of 208 °C. It is isomeric with two other ...
, pyrimidine, or pyrazine
Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2. It is a symmetrical molecule with point group D2h. Pyrazine is less basic than pyridine, pyridazine and pyrimidine. It is a ''"deliquescent crystal or wax-li ...
.
Production
Four chemical processes contribute to industrial benzene production:catalytic reforming
Catalytic reforming is a chemical process used to convert petroleum refinery naphthas distilled from crude oil (typically having low octane ratings) into high-octane liquid products called reformates, which are premium blending stocks for high-oc ...
, toluene hydrodealkylation, toluene disproportionation, and steam cracking etc. According to the ATSDR
The Agency for Toxic Substances and Disease Registry (ATSDR) is a federal public health agency within the United States Department of Health and Human Services. The agency focuses on minimizing human health risks associated with exposure to haza ...
Toxicological Profile for benzene, between 1978 and 1981, catalytic reformates accounted for approximately 44–50% of the total U.S benzene production.
Catalytic reforming
In catalytic reforming, a mixture ofhydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s with boiling points between 60 and 200 °C is blended with hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
gas and then exposed to a bifunctional
In organic chemistry, when a single organic molecule has two different functional groups, it is called a bifunctional molecule . A bifunctional molecule has the properties of two different types of functional groups, such as an alcohol (), amide ( ...
platinum chloride or rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
chloride catalyst at 500–525 °C and pressures ranging from 8–50 atm. Under these conditions, aliphatic hydrocarbons form rings and lose hydrogen to become aromatic hydrocarbons. The aromatic products of the reaction are then separated from the reaction mixture (or reformate) by extraction with any one of a number of solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
s, including diethylene glycol
Diethylene glycol (DEG) is an organic compound with the formula (HOCH2CH2)2O. It is a colorless, practically odorless, and hygroscopic liquid with a sweetish taste. It is a four carbon dimer of ethylene glycol. It is miscible in water, alcohol, ...
or sulfolane, and benzene is then separated from the other aromatics by distillation. The extraction step of aromatics from the reformate is designed to produce aromatics with lowest non-aromatic components. Recovery of the aromatics, commonly referred to as BTX (benzene, toluene and xylene isomers), involves such extraction and distillation steps.
In similar fashion to this catalytic reforming, UOP and BP commercialized a method from LPG (mainly propane and butane) to aromatics.
Toluene hydrodealkylation
Toluene hydrodealkylation converts toluene to benzene. In this hydrogen-intensive process, toluene is mixed with hydrogen, then passed over a chromium, molybdenum, or platinum oxide catalyst at 500–650 °C and 20–60 atm pressure. Sometimes, higher temperatures are used instead of a catalyst (at the similar reaction condition). Under these conditions, toluene undergoes dealkylation to benzene and methane: :biphenyl
Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one ...
(aka diphenyl) at higher temperature:
:2 +
If the raw material stream contains much non-aromatic components (paraffins or naphthenes), those are likely decomposed to lower hydrocarbons such as methane, which increases the consumption of hydrogen.
A typical reaction yield exceeds 95%. Sometimes, xylenes and heavier aromatics are used in place of toluene, with similar efficiency.
This is often called "on-purpose" methodology to produce benzene, compared to conventional BTX (benzene-toluene-xylene) extraction processes.
Toluene disproportionation
Toluene disproportionation (TDP) is the conversion of toluene to benzene and xylene. Given that demand for ''para''-xylene ( ''p''-xylene) substantially exceeds demand for other xylene isomers, a refinement of the TDP process called Selective TDP (STDP) may be used. In this process, the xylene stream exiting the TDP unit is approximately 90% ''p''-xylene. In some systems, even the benzene-to-xylenes ratio is modified to favor xylenes.Steam cracking
Steam cracking is the process for producing ethylene and other alkenes from aliphatic hydrocarbons. Depending on the feedstock used to produce the olefins, steam cracking can produce a benzene-rich liquid by-product called '' pyrolysis gasoline''. Pyrolysis gasoline can be blended with other hydrocarbons as a gasoline additive, or routed through an extraction process to recover BTX aromatics (benzene, toluene and xylenes).Other methods
Although of no commercial significance, many other routes to benzene exist. Phenol and halobenzenes can be reduced with metals. Benzoic acid and its salts undergo decarboxylation to benzene. The reaction of the diazonium compound derived fromaniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starti ...
with hypophosphorus acid gives benzene. Alkyne trimerisation of acetylene gives benzene. Complete decarboxylation of mellitic acid
Mellitic acid, also called graphitic acid or benzenehexacarboxylic acid, is an acid first discovered in 1799 by Martin Heinrich Klaproth in the mineral mellite (honeystone), which is the aluminium salt of the acid. It crystallizes in fine silky ne ...
gives benzene.
Uses
Benzene is used mainly as an intermediate to make other chemicals, above all ethylbenzene (and otheralkylbenzenes
The alkylbenzenes are derivatives of benzene, in which one or more hydrogen atoms are replaced by alkyl groups of different sizes. They are a subset of the aromatic hydrocarbons. The simplest member is toluene, in which a hydrogen atom of the benze ...
), cumene
Cumene (isopropylbenzene) is an organic compound that contains a benzene ring with an isopropyl substituent. It is a constituent of crude oil and refined fuels. It is a flammable colorless liquid that has a boiling point of 152 °C. Near ...
, cyclohexane, and nitrobenzene. In 1988 it was reported that two-thirds of all chemicals on the American Chemical Society's lists contained at least one benzene ring. More than half of the entire benzene production is processed into ethylbenzene, a precursor to styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
, which is used to make polymers and plastics like polystyrene. Some 20% of the benzene production is used to manufacture cumene, which is needed to produce phenol and acetone for resins and adhesives. Cyclohexane consumes around 10% of the world's benzene production; it is primarily used in the manufacture of nylon fibers, which are processed into textiles and engineering plastics. Smaller amounts of benzene are used to make some types of rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, an ...
s, lubricants, dyes, detergents, drugs, explosives, and pesticides. In 2013, the biggest consumer country of benzene was China, followed by the USA. Benzene production is currently expanding in the Middle East and in Africa, whereas production capacities in Western Europe and North America are stagnating.
Toluene is now often used as a substitute for benzene, for instance as a fuel additive. The solvent-properties of the two are similar, but toluene is less toxic and has a wider liquid range. Toluene is also processed into benzene.
Cumene
Cumene (isopropylbenzene) is an organic compound that contains a benzene ring with an isopropyl substituent. It is a constituent of crude oil and refined fuels. It is a flammable colorless liquid that has a boiling point of 152 °C. Near ...
rect 665 795 1062 942 Cyclohexane
rect 665 1161 1062 1317 Aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starti ...
rect 665 1533 1062 1686 Chlorobenzene
Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.
Uses
Historical
The major use of chlorob ...
rect 1215 345 1614 495 Acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscib ...
rect 1215 636 1614 783 Phenol
rect 1764 57 2163 210 Styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
rect 1764 432 2163 585 Bisphenol A
rect 1764 1083 2163 1233 Adipic acid
rect 1764 1332 2163 1482 Caprolactam
rect 2313 57 2712 207 Polystyrene
rect 2313 315 2712 462 Polycarbonate
rect 2313 570 2712 717 Epoxy resin
rect 2313 822 2712 975 Phenolic resin
Phenol formaldehyde resins (PF) or phenolic resins (also infrequently called phenoplasts) are synthetic polymers obtained by the reaction of phenol or substituted phenol with formaldehyde. Used as the basis for Bakelite, PFs were the first commerc ...
rect 2313 1083 2712 1233 Nylon 6-6
rect 2313 1335 2712 1485 Nylon 6
desc bottom-left
Component of gasoline
As agasoline
Gasoline (; ) or petrol (; ) (see ) is a transparent, petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines (also known as petrol engines). It consists mostly of organi ...
(petrol) additive, benzene increases the octane rating and reduces knocking. As a consequence, gasoline often contained several percent benzene before the 1950s, when tetraethyl lead
Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula Pb( C2H5)4. It is a fuel additive, first being mixed with gasoline beginning in the 1920s as a patented octane rating booster that al ...
replaced it as the most widely used antiknock additive. With the global phaseout of leaded gasoline, benzene has made a comeback as a gasoline additive in some nations. In the United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territori ...
, concern over its negative health effects and the possibility of benzene entering the groundwater
Groundwater is the water present beneath Earth's surface in rock and soil pore spaces and in the fractures of rock formations. About 30 percent of all readily available freshwater in the world is groundwater. A unit of rock or an unconsolidated ...
has led to stringent regulation of gasoline's benzene content, with limits typically around 1%. European petrol specifications now contain the same 1% limit on benzene content. The United States Environmental Protection Agency
The Environmental Protection Agency (EPA) is an independent executive agency of the United States federal government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it ...
introduced new regulations in 2011 that lowered the benzene content in gasoline to 0.62%.
In many European languages, the word for petroleum or gasoline is an exact cognate of "benzene".
Reactions
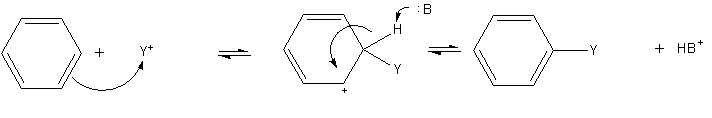
The most common reactions of benzene involve substitution of a proton by other groups.Electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic n ...
is a general method of derivatizing benzene. Benzene is sufficiently nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
that it undergoes substitution by acylium
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an alkyl group (). In organic chemistry, the acyl group (IUPAC n ...
ions and alkyl carbocations to give substituted derivatives.
: The most widely practiced example of this reaction is the ethylation of benzene.
::
The most widely practiced example of this reaction is the ethylation of benzene.
:: Approximately 24,700,000 tons were produced in 1999. Highly instructive but of far less industrial significance is the Friedel-Crafts alkylation of benzene (and many other aromatic rings) using an
Approximately 24,700,000 tons were produced in 1999. Highly instructive but of far less industrial significance is the Friedel-Crafts alkylation of benzene (and many other aromatic rings) using an alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely us ...
in the presence of a strong Lewis acid catalyst. Similarly, the Friedel-Crafts acylation is a related example of electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic n ...
. The reaction involves the acylation
In chemistry, acylation (or alkanoylation) is the chemical reaction in which an acyl group () is added to a compound. The compound providing the acyl group is called the acylating agent.
Because they form a strong electrophile when treated with ...
of benzene (or many other aromatic rings) with an acyl chloride using a strong Lewis acid catalyst such as aluminium chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms hexahydrate with the formula , containing six water molecules of hydration. Both are colourless crystals, but samples are often contam ...
or Iron(III) chloride.
Sulfonation, chlorination, nitration
Using electrophilic aromatic substitution, many functional groups are introduced onto the benzene framework.Sulfonation Aromatic sulfonation is an organic reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid functional group in an electrophilic aromatic substitution. Aryl sulfonic acids are used as detergents, dye, and drugs.
Stoichiometry a ...
of benzene involves the use of oleum, a mixture of sulfuric acid with sulfur trioxide. Sulfonated benzene derivatives are useful detergents. In nitration
In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols an ...
, benzene reacts with nitronium ions (NO2+), which is a strong electrophile produced by combining sulfuric and nitric acids. Nitrobenzene is the precursor to aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starti ...
. Chlorination is achieved with chlorine to give chlorobenzene
Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.
Uses
Historical
The major use of chlorob ...
in the presence of a Lewis acid catalyst such as aluminium tri-chloride.
Hydrogenation
Viahydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organ ...
, benzene and its derivatives convert to cyclohexane and derivatives. This reaction is achieved by the use of high pressures of hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
in the presence of heterogeneous catalysts, such as finely divided nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
. Whereas alkenes can be hydrogenated near room temperatures, benzene and related compounds are more reluctant substrates, requiring temperatures >100 °C. This reaction is practiced on a large scale industrially. In the absence of the catalyst, benzene is impervious to hydrogen. Hydrogenation cannot be stopped to give cyclohexene or cyclohexadienes as these are superior substrates. Birch reduction, a non catalytic process, however selectively hydrogenates benzene to the diene.
Metal complexes
Benzene is an excellent ligand in the organometallic chemistry of low-valent metals. Important examples include the sandwich and half-sandwich complexes, respectively, Cr(C6H6)2 and uCl2(C6H6)sub>2.Health effects
American Petroleum Institute
The American Petroleum Institute (API) is the largest U.S. trade association for the oil and natural gas industry. It claims to represent nearly 600 corporations involved in production, refinement, distribution, and many other aspects of the ...
(API) stated in 1948 that "it is generally considered that the only absolutely safe concentration for benzene is zero". There is no safe exposure level; even tiny amounts can cause harm. The US Department of Health and Human Services
The United States Department of Health and Human Services (HHS) is a cabinet-level executive branch department of the U.S. federal government created to protect the health of all Americans and providing essential human services. Its motto is ...
(DHHS) classifies benzene as a human carcinogen. Long-term exposure to excessive levels of benzene in the air causes leukemia
Leukemia ( also spelled leukaemia and pronounced ) is a group of blood cancers that usually begin in the bone marrow and result in high numbers of abnormal blood cells. These blood cells are not fully developed and are called ''blasts'' or ...
, a potentially fatal cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
of the blood-forming organs. In particular, acute myeloid leukemia
Acute myeloid leukemia (AML) is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal cells that build up in the bone marrow and blood and interfere with normal blood cell production. Symptoms may inclu ...
or acute nonlymphocytic leukemia (AML & ANLL) is caused by benzene. IARC rated benzene as "known to be carcinogenic to humans" (Group 1).
As benzene is ubiquitous in gasoline and hydrocarbon fuels that are in use everywhere, human exposure to benzene is a global health problem. Benzene targets the liver, kidney, lung, heart and brain and can cause DNA strand breaks and chromosomal damage. Benzene causes cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
in animals including humans. Benzene has been shown to cause cancer in both sexes of multiple species of laboratory animals exposed via various routes.
Exposure to benzene
According to the Agency for Toxic Substances and Disease Registry (ATSDR) (2007), benzene is both a synthetically-made and naturally occurring chemical from processes that include: volcanic eruptions, wild fires, synthesis of chemicals such as phenol, production of synthetic fibers, and fabrication ofrubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, an ...
s, lubricants, pesticides, medications, and dyes. The major sources of benzene exposure are tobacco smoke, automobile service stations, exhaust from motor vehicles, and industrial emissions; however, ingestion and dermal absorption of benzene can also occur through contact with contaminated water. Benzene is hepatically metabolized and excreted in the urine. Measurement of air and water levels of benzene is accomplished through collection via activated charcoal tubes, which are then analyzed with a gas chromatograph. The measurement of benzene in humans can be accomplished via urine, blood, and breath tests; however, all of these have their limitations because benzene is rapidly metabolized in the human body.
Exposure to benzene may lead progressively to aplastic anemia, leukaemia
Leukemia ( also spelled leukaemia and pronounced ) is a group of blood cancers that usually begin in the bone marrow and result in high numbers of abnormal blood cells. These blood cells are not fully developed and are called ''blasts'' or ...
, and multiple myeloma.
OSHA
OSHA or Osha may refer to:
Work
* Occupational Safety and Health Administration, a federal agency of the United States that regulates workplace safety and health
* Occupational Safety and Health Act (United States) of 1970, a federal law in the Un ...
regulates levels of benzene in the workplace. The maximum allowable amount of benzene in workroom air during an 8-hour workday, 40-hour workweek is 1 ppm. As benzene can cause cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
, NIOSH recommends that all workers wear special breathing equipment when they are likely to be exposed to benzene at levels exceeding the recommended (8-hour) exposure limit of 0.1 ppm.
Benzene exposure limits
TheUnited States Environmental Protection Agency
The Environmental Protection Agency (EPA) is an independent executive agency of the United States federal government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it ...
has set a maximum contaminant level for benzene in drinking water
Drinking water is water that is used in drink or food preparation; potable water is water that is safe to be used as drinking water. The amount of drinking water required to maintain good health varies, and depends on physical activity level, a ...
at 0.0005 mg/L (5 ppb), as promulgated via the U.S. National Primary Drinking Water Regulations. This regulation is based on preventing benzene leukemogenesis. The maximum contaminant level goal ( MCLG), a nonenforceable health goal that would allow an adequate margin of safety for the prevention of adverse effects, is zero benzene concentration in drinking water. The EPA requires that spills or accidental releases into the environment of 10 pounds (4.5 kg) or more of benzene be reported.
The U.S. Occupational Safety and Health Administration (OSHA) has set a permissible exposure limit of 1 part of benzene per million parts of air (1 ppm) in the workplace during an 8-hour workday, 40-hour workweek. The short term exposure limit for airborne benzene is 5 ppm for 15 minutes. These legal limits were based on studies demonstrating compelling evidence of health risk to workers exposed to benzene. The risk from exposure to 1 ppm for a working lifetime has been estimated as 5 excess leukemia deaths per 1,000 employees exposed. (This estimate assumes no threshold for benzene's carcinogenic effects.) OSHA has also established an action level of 0.5 ppm to encourage even lower exposures in the workplace.
The U.S. National Institute for Occupational Safety and Health
The National Institute for Occupational Safety and Health (NIOSH, ) is the United States federal agency responsible for conducting research and making recommendations for the prevention of work-related injury and illness. NIOSH is part of the C ...
(NIOSH) revised the Immediately Dangerous to Life and Health (IDLH) concentration for benzene to 500 ppm. The current NIOSH definition for an IDLH condition, as given in the NIOSH Respirator Selection Logic, is one that poses a threat of exposure to airborne contaminants when that exposure is likely to cause death or immediate or delayed permanent adverse health effects or prevent escape from such an environment. The purpose of establishing an IDLH value is (1) to ensure that the worker can escape from a given contaminated environment in the event of failure of the respiratory protection equipment and (2) is considered a maximum level above which only a highly reliable breathing apparatus providing maximum worker protection is permitted. publication No. 2005-100. In September 1995, NIOSH issued a new policy for developing recommended exposure limit
A recommended exposure limit (REL) is an occupational exposure limit that has been recommended by the United States National Institute for Occupational Safety and Health. The REL is a level that NIOSH believes would be protective of worker safet ...
s (RELs) for substances, including carcinogens. As benzene can cause cancer, NIOSH recommends that all workers wear special breathing equipment when they are likely to be exposed to benzene at levels exceeding the REL (10-hour) of 0.1 ppm. The NIOSH short-term exposure limit (STEL – 15 min) is 1 ppm.
American Conference of Governmental Industrial Hygienists (ACGIH) adopted Threshold Limit Values (TLVs) for benzene at 0.5 ppm TWA and 2.5 ppm STEL.
Toxicology
Biomarkers of exposure
Several tests can determine exposure to benzene. Benzene itself can be measured in breath, blood or urine, but such testing is usually limited to the first 24 hours post-exposure due to the relatively rapid removal of the chemical by exhalation or biotransformation. Most people in developed countries have measureable baseline levels of benzene and other aromatic petroleum hydrocarbons in their blood. In the body, benzene is enzymatically converted to a series of oxidation products includingmuconic acid
Muconic acid is a dicarboxylic acid. There are three isomeric forms designated ''trans,trans''-muconic acid, ''cis,trans''-muconic acid, and ''cis,cis''-muconic acid which differ by the geometry around the double bonds. Its name is derived from ...
, phenylmercapturic acid, phenol, catechol
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amoun ...
, hydroquinone and 1,2,4-trihydroxybenzene. Most of these metabolites have some value as biomarkers of human exposure, since they accumulate in the urine in proportion to the extent and duration of exposure, and they may still be present for some days after exposure has ceased. The current ACGIH biological exposure limits for occupational exposure are 500 μg/g creatinine for muconic acid and 25 μg/g creatinine for phenylmercapturic acid in an end-of-shift urine specimen.
Biotransformations
Even if it is not a common substrate for metabolism, benzene can be oxidized by bothbacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of prokaryotic microorganisms. Typically a few micrometr ...
and eukaryotes. In bacteria, dioxygenase enzyme can add an oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
to the ring, and the unstable product is immediately reduced (by NADH
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an aden ...
) to a cyclic diol with two double bonds, breaking the aromaticity. Next, the diol is newly reduced by NADH to catechol
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amoun ...
. The catechol is then metabolized to acetyl CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for ...
and succinyl CoA, used by organisms mainly in the citric acid cycle for energy production.
The pathway for the metabolism of benzene is complex and begins in the liver. Several enzymes are involved. These include cytochrome P450
Cytochromes P450 (CYPs) are a superfamily of enzymes containing heme as a cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various co ...
2E1 (CYP2E1), quinine oxidoreductase (NQ01 or DT-diaphorase or NAD(P)H dehydrogenase (quinone 1)), GSH, and myeloperoxidase (MPO). CYP2E1 is involved at multiple steps: converting benzene to oxepin
Oxepin is an oxygen-containing heterocycle consisting of a seven-membered ring with three double bonds. The parent C6H6O exists as an equilibrium mixture with benzene oxide. The oxepin–benzene oxide equilibrium is affected by the ring substi ...
(benzene oxide), phenol to hydroquinone, and hydroquinone to both benzenetriol and catechol
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amoun ...
. Hydroquinone, benzenetriol and catechol are converted to polyphenols. In the bone marrow, MPO converts these polyphenols to benzoquinones. These intermediates and metabolites induce genotoxicity by multiple mechanisms including inhibition of topoisomerase II
Type II topoisomerases are topoisomerases that cut both strands of the DNA helix simultaneously in order to manage DNA tangles and supercoils. They use the hydrolysis of ATP, unlike Type I topoisomerase. In this process, these enzymes change th ...
(which maintains chromosome structure), disruption of microtubules (which maintains cellular structure and organization), generation of oxygen free radicals (unstable species) that may lead to point mutations, increasing oxidative stress, inducing DNA strand breaks, and altering DNA methylation (which can affect gene expression). NQ01 and GSH shift metabolism away from toxicity. NQ01 metabolizes benzoquinone Benzoquinone (C6H4O2) is a quinone with a single benzene ring. There are 2 (out of 3 hypothetical) benzoquinones:
* 1,4-Benzoquinone, most commonly, right image (also ''para''-benzoquinone, ''p''-benzoquinone, ''para''-quinone, or just quinone)
* 1 ...
toward polyphenols
Polyphenols () are a large family of naturally occurring organic compounds characterized by multiples of phenol units. They are abundant in plants and structurally diverse. Polyphenols include flavonoids, tannic acid, and ellagitannin, some of ...
(counteracting the effect of MPO). GSH is involved with the formation of } phenylmercapturic acid.
Genetic polymorphisms in these enzymes may induce loss of function or gain of function. For example, mutations in CYP2E1 increase activity and result in increased generation of toxic metabolites. NQ01 mutations result in loss of function and may result in decreased detoxification. Myeloperoxidase mutations result in loss of function and may result in decreased generation of toxic metabolites. GSH mutations or deletions result in loss of function and result in decreased detoxification. These genes may be targets for genetic screening for susceptibility to benzene toxicity.
Molecular toxicology
The paradigm of toxicological assessment of benzene is shifting towards the domain of molecular toxicology as it allows understanding of fundamental biological mechanisms in a better way.Glutathione
Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, pe ...
seems to play an important role by protecting against benzene-induced DNA breaks and it is being identified as a new biomarker for exposure and effect. Benzene causes chromosomal aberrations in the peripheral blood leukocytes and bone marrow explaining the higher incidence of leukemia and multiple myeloma caused by chronic exposure. These aberrations can be monitored using fluorescent in situ hybridization (FISH) with DNA probes to assess the effects of benzene along with the hematological tests as markers of hematotoxicity. Benzene metabolism involves enzymes coded for by polymorphic genes. Studies have shown that genotype at these loci may influence susceptibility to the toxic effects of benzene exposure. Individuals carrying variant of NAD(P)H:quinone oxidoreductase 1 (NQO1), microsomal epoxide hydrolase (EPHX) and deletion of the glutathione S-transferase T1 (GSTT1) showed a greater frequency of DNA single-stranded breaks.
Biological oxidation and carcinogenic activity
One way of understanding the carcinogenic effects of benzene is by examining the products of biological oxidation. Pure benzene, for example, oxidizes in the body to produce an epoxide, benzene oxide, which is not excreted readily and can interact with DNA to produce harmful mutations.Routes of exposure
Inhalation
Outdoor air may contain low levels of benzene from automobile service stations, wood smoke, tobacco smoke, the transfer of gasoline, exhaust from motor vehicles, and industrial emissions. About 50% of the entire nationwide (United States) exposure to benzene results from smoking tobacco or from exposure to tobacco smoke. After smoking 32 cigarettes per day, the smoker would take in about 1.8 milligrams (mg) of benzene. This amount is about 10 times the average daily intake of benzene by nonsmokers. Inhaled benzene is primarily expelled unchanged through exhalation. In a human study 16.4 to 41.6% of retained benzene was eliminated through the lungs within five to seven hours after a two- to three-hour exposure to 47 to 110 ppm and only 0.07 to 0.2% of the remaining benzene was excreted unchanged in the urine. After exposure to 63 to 405 mg/m3 of benzene for 1 to 5 hours, 51 to 87% was excreted in the urine as phenol over a period of 23 to 50 hours. In another human study, 30% of absorbed dermally applied benzene, which is primarily metabolized in the liver, was excreted as phenol in the urine.Exposure from soft drinks
Under specific conditions and in the presence of other chemicals benzoic acid (a preservative) and ascorbic acid (Vitamin C) may interact to produce benzene. In March 2006, the official Food Standards Agency inUnited Kingdom
The United Kingdom of Great Britain and Northern Ireland, commonly known as the United Kingdom (UK) or Britain, is a country in Europe, off the north-western coast of the European mainland, continental mainland. It comprises England, Scotlan ...
conducted a survey of 150 brands of soft drinks. It found that four contained benzene levels above World Health Organization
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. The WHO Constitution states its main objective as "the attainment by all peoples of the highest possible level of ...
limits. The affected batches were removed from sale. Similar problems were reported by the FDA in the United States.
Contamination of water supply
In 2005, the water supply to the city of Harbin in China with a population of almost nine million people, was cut off because of a major benzene exposure. Benzene leaked into the Songhua River, which supplies drinking water to the city, after an explosion at a China National Petroleum Corporation (CNPC) factory in the city of Jilin on 13 November 2005. When plastic water pipes are subject to high heat, the water may be contaminated with benzene.Genocide
The Nazis used benzene administered viainjection
Injection or injected may refer to:
Science and technology
* Injective function, a mathematical function mapping distinct arguments to distinct values
* Injection (medicine), insertion of liquid into the body with a syringe
* Injection, in broadca ...
as one of their many methods for killing.
See also
* BTEX * '' Industrial Union Department v. American Petroleum Institute'' * Six-membered aromatic rings with one carbon replaced by another element:borabenzene
Borabenzene is a hypothetical organoboron compound with the formula C5H5B. Unlike the related but highly stable benzene molecule, borabenzene would be electron-deficient. Related derivatives are the boratabenzene anions, including the parent 5H ...
, silabenzene
A silabenzene is a heteroaromatic compound containing one or more silicon atoms instead of carbon atoms in benzene. A single substitution gives silabenzene proper; additional substitutions give a disilabenzene (3 theoretical isomers), trisilaben ...
, germabenzene, stannabenzene, pyridine, phosphorine, arsabenzene
Arsabenzene (IUPAC name: arsinine) is an organoarsenic heterocyclic compound with the chemical formula C5H5As. It belongs to a group of compounds called heteroarenes that have the general formula C5H5E (E= N, P, As, Sb, Bi).
This air sensitive li ...
, bismabenzene, pyrylium, thiopyrylium
Thiopyrylium is a cation with the chemical formula C5H5S+. It is analogous to the pyrylium cation with the oxygen atom replaced by a sulfur atom.
Thiopyrylium salts are less reactive than the analogous pyrylium salts due to the higher polarizab ...
, selenopyrylium, telluropyrylium
Explanatory notes
References
External links
Benzene
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
International Chemical Safety Card 0015
*
Dept. of Health and Human Services: TR-289: Toxicology and Carcinogenesis Studies of Benzene
Video Podcast
of Sir John Cadogan giving a lecture on Benzene since Faraday, in 1991
Substance profile
*
NLM Hazardous Substances Databank – Benzene
{{Authority control Annulenes Aromatic hydrocarbons Aromatic solvents Carcinogens Commodity chemicals GABAA receptor positive allosteric modulators Hazardous air pollutants Hydrocarbon solvents IARC Group 1 carcinogens Immunotoxins Mutagens Occupational safety and health Petrochemicals Simple aromatic rings Six-membered rings Soil contamination Sweet-smelling chemicals Teratogens