Photostability on:
[Wikipedia]
[Google]
[Amazon]
 Photochemistry is the branch of
Photochemistry is the branch of
 Alternatively, it is possible for the excited state S1 to undergo spin inversion and to generate a triplet excited state T1 having two unpaired electrons with the same spin. This violation of the spin selection rule is possible by
Alternatively, it is possible for the excited state S1 to undergo spin inversion and to generate a triplet excited state T1 having two unpaired electrons with the same spin. This violation of the spin selection rule is possible by
 Photochemical reactions require a light source that emits wavelengths corresponding to an electronic transition in the reactant. In the early experiments (and in everyday life), sunlight was the light source, although it is polychromatic.
Photochemical reactions require a light source that emits wavelengths corresponding to an electronic transition in the reactant. In the early experiments (and in everyday life), sunlight was the light source, although it is polychromatic.  The emitted light must of course reach the targeted
The emitted light must of course reach the targeted
 Photochemistry is the branch of
Photochemistry is the branch of chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nanometer, nm (with a corresponding frequency around 30 Hertz, PHz) to 400 nm (750 Hertz, THz), shorter than that of visible light, but longer than ...
(wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tro ...
from 100 to 400 nm), visible light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 te ...
(400–750 nm) or infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around ...
radiation (750–2500 nm).
In nature, photochemistry is of immense importance as it is the basis of photosynthesis, vision, and the formation of vitamin D
Vitamin D is a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, and many other biological effects. In humans, the most important compounds in this group are vitamin D3 (c ...
with sunlight. Photochemical reactions proceed differently than temperature-driven reactions. Photochemical paths access high energy intermediates that cannot be generated thermally, thereby overcoming large activation barriers in a short period of time, and allowing reactions otherwise inaccessible by thermal processes. Photochemistry can also be destructive, as illustrated by the photodegradation Photodegradation is the alteration of materials by light. Commonly, the term is used loosely to refer to the combined action of sunlight and air, which cause oxidation and hydrolysis. Often photodegradation is intentionally avoided, since it destroy ...
of plastics.
Concept
Grotthuss–Draper law and Stark-Einstein law
Photoexcitation
Photoexcitation is the production of an excited state of a quantum system by photon absorption. The excited state originates from the interaction between a photon and the quantum system. Photons carry energy that is determined by the wavelengths ...
is the first step in a photochemical process where the reactant is elevated to a state of higher energy, an excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to a ...
.
The first law of photochemistry, known as the Grotthuss–Draper law
Photoelectrochemical processes are processes in photoelectrochemistry; they usually involve transforming light into other forms of energy.
These processes apply to photochemistry, optically pumped lasers, sensitized solar cells, luminescence, and ...
(for chemists Theodor Grotthuss
Freiherr Christian Johann Dietrich Theodor von Grotthuss (20 January 1785 – 26 March 1822) was a Baltic German scientist known for establishing the first theory of electrolysis in 1806 and formulating the first law of photochemistry in 1817. His ...
and John W. Draper), states that light must be absorbed by a chemical substance in order for a photochemical reaction Organic photochemistry encompasses organic reactions that are induced by the action of light. The absorption of ultraviolet light by organic molecules often leads to reactions. In the earliest days, sunlight was employed, while in more modern times ...
to take place. According to the second law of photochemistry, known as the Stark-Einstein law (for physicists Johannes Stark
Johannes Stark (, 15 April 1874 – 21 June 1957) was a German physicist who was awarded the Nobel Prize in Physics in 1919 "for his discovery of the Doppler effect in canal rays and the splitting of spectral lines in electric fields". This phen ...
and Albert Einstein
Albert Einstein ( ; ; 14 March 1879 – 18 April 1955) was a German-born theoretical physicist, widely acknowledged to be one of the greatest and most influential physicists of all time. Einstein is best known for developing the theory ...
), for each photon of light absorbed by a chemical system, no more than one molecule is activated for a photochemical reaction, as defined by the quantum yield The quantum yield (Φ) of a radiation-induced process is the number of times a specific event occurs per photon absorbed by the system.
Applications
Fluorescence spectroscopy
The fluorescence quantum yield is defined as the ratio of the numb ...
.
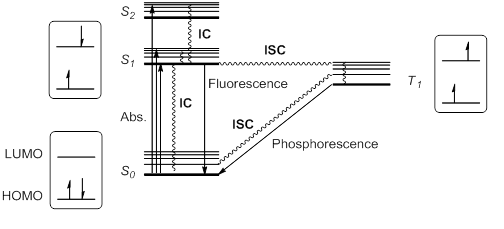
Fluorescence and phosphorescence
When a molecule or atom in theground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
(S0) absorbs light, one electron is excited to a higher orbital level. This electron maintains its spin
Spin or spinning most often refers to:
* Spinning (textiles), the creation of yarn or thread by twisting fibers together, traditionally by hand spinning
* Spin, the rotation of an object around a central axis
* Spin (propaganda), an intentionally b ...
according to the spin selection rule; other transitions would violate the law of conservation of angular momentum
In physics, angular momentum (rarely, moment of momentum or rotational momentum) is the rotational analog of linear momentum. It is an important physical quantity because it is a conserved quantity—the total angular momentum of a closed system ...
.
The excitation to a higher singlet state can be from HOMO
''Homo'' () is the genus that emerged in the (otherwise extinct) genus ''Australopithecus'' that encompasses the extant species ''Homo sapiens'' ( modern humans), plus several extinct species classified as either ancestral to or closely relate ...
to LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
or to a higher orbital, so that singlet excitation states S1, S2, S3… at different energies are possible.
Kasha's rule stipulates that higher singlet states would quickly relax by radiationless decay or internal conversion
Internal conversion is a non-radioactive, atomic decay process where an excited nucleus interacts electromagnetically with one of the orbital electrons of an atom. This causes the electron to be emitted (ejected) from the atom. Thus, in internal ...
(IC) to S1. Thus, S1 is usually, but not always, the only relevant singlet excited state. This excited state S1 can further relax to S0 by IC, but also by an allowed radiative transition from S1 to S0 that emits a photon; this process is called fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
.
 Alternatively, it is possible for the excited state S1 to undergo spin inversion and to generate a triplet excited state T1 having two unpaired electrons with the same spin. This violation of the spin selection rule is possible by
Alternatively, it is possible for the excited state S1 to undergo spin inversion and to generate a triplet excited state T1 having two unpaired electrons with the same spin. This violation of the spin selection rule is possible by intersystem crossing Intersystem crossing (ISC) is an isoenergetic radiationless process involving a transition between the two electronic states with different spin multiplicity.
Excited Singlet and Triplet States
When an electron in a molecule with a singlet ground ...
(ISC) of the vibrational and electronic levels of S1 and T1. According to Hund's rule of maximum multiplicity
Hund's rule of maximum multiplicity is a rule based on observation of atomic spectra, which is used to predict the ground state of an atom or molecule with one or more open electronic shells. The rule states that for a given electron configurati ...
, this T1 state would be somewhat more stable than S1.
This triplet state can relax to the ground state S0 by radiationless IC or by a radiation pathway called phosphorescence
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluo ...
. This process implies a change of electronic spin, which is forbidden by spin selection rules, making phosphorescence (from T1 to S0) much slower than fluorescence (from S1 to S0). Thus, triplet states generally have longer lifetimes than singlet states.
These transitions are usually summarized in a state energy diagram or Jablonski diagram Jabłoński (Polish pronunciation: ; feminine: Jabłońska; plural: Jabłońscy) is a Polish surname derived from the noun ''jabłoń'' (''apple tree''). It appears in various forms when transliterated from Cyrillic alphabets.
People
* Aleksan ...
, the paradigm of molecular photochemistry.
These excited species, either S1 or T1, have a half empty low-energy orbital, and are consequently more oxidizing than the ground state. But at the same time, they have an electron in a high energy orbital, and are thus more reducing. In general, excited species are prone to participate in electron transfer processes.
Experimental set-up
Mercury-vapor lamp
A mercury-vapor lamp is a gas-discharge lamp that uses an electric arc through vaporized mercury to produce light. The arc discharge is generally confined to a small fused quartz arc tube mounted within a larger soda lime or borosilicate glass ...
s are more common in the laboratory. Low pressure mercury vapor lamps mainly emit at 254 nm. For polychromatic sources, wavelength ranges can be selected using filters. Alternatively, laser beams are usually monochromatic (although two or more wavelengths can be obtained using nonlinear optics) and LED
A light-emitting diode (LED) is a semiconductor Electronics, device that Light#Light sources, emits light when Electric current, current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy i ...
s have a relatively narrowband that can be efficiently used, as well as Rayonet lamps, to get approximately monochromatic beams.
 The emitted light must of course reach the targeted
The emitted light must of course reach the targeted functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
without being blocked by the reactor, medium, or other functional groups present. For many applications, quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical form ...
is used for the reactors as well as to contain the lamp. Pyrex
Pyrex (trademarked as ''PYREX'' and ''pyrex'') is a brand introduced by Corning Inc. in 1915 for a line of clear, low-thermal-expansion borosilicate glass used for laboratory glassware and kitchenware. It was later expanded to include kitchenw ...
absorbs at wavelengths shorter than 275 nm. The solvent is an important experimental parameter. Solvents are potential reactants and for this reason, chlorinated solvents are avoided because the C-Cl bond can lead to chlorination Chlorination may refer to:
* Chlorination reaction
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transform ...
of the substrate. Strongly absorbing solvents prevent photons from reaching the substrate. Hydrocarbon solvents absorb only at short wavelengths and are thus preferred for photochemical experiments requiring high energy photons. Solvents containing unsaturation absorb at longer wavelengths and can usefully filter out short wavelengths. For example, cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
and acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscib ...
"cut off" (absorb strongly) at wavelengths shorter than 215 and 330 nm, respectively.
Photochemistry in combination with
flow chemistry
In flow chemistry, a chemical reaction is run in a continuously flowing stream rather than in batch production. In other words, pumps move fluid into a reactor, and where tubes join one another, the fluids contact one another. If these fluids are ...
Continuous flow photochemistry offers multiple advantages over batch photochemistry. Photochemical reactions are driven by the number of photons that are able to activate molecules causing the desired reaction. The large surface area to volume ratio of a microreactor maximizes the illumination, and at the same time allows for efficient cooling, which decreases the thermal side products.
Principles
In the case of photochemical reactions, light provides theactivation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
. Simplistically, light is one mechanism for providing the activation energy required for many reactions. If laser light is employed, it is possible to selectively excite a molecule so as to produce a desired electronic and vibrational state. Equally, the emission from a particular state may be selectively monitored, providing a measure of the population of that state. If the chemical system is at low pressure, this enables scientists to observe the energy distribution of the products of a chemical reaction before the differences in energy have been smeared out and averaged by repeated collisions.
The absorption of a photon of light by a reactant molecule may also permit a reaction to occur not just by bringing the molecule to the necessary activation energy, but also by changing the symmetry of the molecule's electronic configuration, enabling an otherwise inaccessible reaction path, as described by the Woodward–Hoffmann selection rules. A 2+2 cycloaddition reaction is one example of a pericyclic reaction
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overlap ...
that can be analyzed using these rules or by the related frontier molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
theory.
Some photochemical reactions are several orders of magnitude faster than thermal reactions; reactions as fast as 10−9 seconds and associated processes as fast as 10−15 seconds are often observed.
The photon can be absorbed directly by the reactant or by a photosensitizer
Photosensitizers produce a physicochemical change in a neighboring molecule by either donating an electron to the substrate or by abstracting a hydrogen atom from the substrate. At the end of this process, the photosensitizer eventually returns to ...
, which absorbs the photon and transfers the energy to the reactant. The opposite process is called quenching
In materials science, quenching is the rapid cooling of a workpiece in water, oil, polymer, air, or other fluids to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such as pha ...
when a photoexcited state is deactivated by a chemical reagent.
Most photochemical transformations occur through a series of simple steps known as primary photochemical processes. One common example of these processes is the excited state proton transfer.
Photochemical reactions
Examples of
photochemical reactions Organic photochemistry encompasses organic reactions that are induced by the action of light. The absorption of ultraviolet light by organic molecules often leads to reactions. In the earliest days, sunlight was employed, while in more modern times ...
*Photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
: plants use solar energy
Solar energy is radiant light and heat from the Sun that is harnessed using a range of technologies such as solar power to generate electricity, solar thermal energy (including solar water heating), and solar architecture. It is an essenti ...
to convert carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
and water into glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using ...
and oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
.
*Human formation of vitamin D
Vitamin D is a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, and many other biological effects. In humans, the most important compounds in this group are vitamin D3 (c ...
by exposure to sunlight.
*Bioluminescence
Bioluminescence is the production and emission of light by living organisms. It is a form of chemiluminescence. Bioluminescence occurs widely in marine vertebrates and invertebrates, as well as in some fungi, microorganisms including some b ...
: ''e.g.'' In fireflies
The Lampyridae are a family (biology), family of Elateroidea, elateroid beetles with more than 2,000 described species, many of which are bioluminescence, light-emitting. They are soft-bodied beetles commonly called fireflies, lightning bugs, ...
, an enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
in the abdomen catalyzes a reaction that produced light.
*Polymerizations started by photoinitiator
A photoinitiator is a molecule that creates reactive species (free radicals, cations or anions) when exposed to radiation (UV or visible). Synthetic photoinitiators are key components in photopolymers (for example, photo-curable coatings, adhesive ...
s, which decompose upon absorbing light to produce the free radicals for radical polymerization
In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks (repeat units). Free radicals can be formed by a number of different mechanis ...
.
*Photodegradation Photodegradation is the alteration of materials by light. Commonly, the term is used loosely to refer to the combined action of sunlight and air, which cause oxidation and hydrolysis. Often photodegradation is intentionally avoided, since it destroy ...
of many substances, e.g. polyvinyl chloride and Fp. Medicine bottles are often made with darkened glass to prevent the drugs from photodegradation.
*Photochemical rearrangements, ''e.g.'' photoisomerisation, hydrogen atom transfer and photochemical electrocyclic reactions.
*Photodynamic therapy
Photodynamic therapy (PDT) is a form of phototherapy involving light and a photosensitizing chemical substance, used in conjunction with molecular oxygen to elicit cell death (phototoxicity).
PDT is popularly used in treating acne. It is used cl ...
: light is used to destroy tumors by the action of singlet oxygen generated by photosensitized reactions of triplet oxygen. Typical photosensitizers include tetraphenylporphyrin
Tetraphenylporphyrin, abbreviated TPP or H2TPP, is a synthetic heterocyclic compound that resembles naturally occurring porphyrins. Porphyrins are dyes and cofactors found in hemoglobin and cytochromes and are related to chlorophyll and vitamin ...
and methylene blue
Methylthioninium chloride, commonly called methylene blue, is a salt used as a dye and as a medication. Methylene blue is a thiazine dye. As a medication, it is mainly used to treat methemoglobinemia by converting the ferric iron in hemoglobin ...
. The resulting singlet oxygen is an aggressive oxidant, capable of converting C-H bonds into C-OH groups.
* Diazo printing process
*Photoresist
A photoresist (also known simply as a resist) is a light-sensitive material used in several processes, such as photolithography and photoengraving, to form a patterned coating on a surface. This process is crucial in the electronic industry.
T ...
technology, used in the production of microelectronic
Microelectronics is a subfield of electronics. As the name suggests, microelectronics relates to the study and manufacture (or microfabrication) of very small electronic designs and components. Usually, but not always, this means micrometre-sc ...
components.
*Vision
Vision, Visions, or The Vision may refer to:
Perception Optical perception
* Visual perception, the sense of sight
* Visual system, the physical mechanism of eyesight
* Computer vision, a field dealing with how computers can be made to gain un ...
is initiated by a photochemical reaction of rhodopsin
Rhodopsin, also known as visual purple, is a protein encoded by the RHO gene and a G-protein-coupled receptor (GPCR). It is the opsin of the rod cells in the retina and a light-sensitive receptor protein that triggers visual phototransduction ...
.
*Toray
is a multinational corporation headquartered in Japan that specializes in industrial products centered on technologies in organic synthetic chemistry, polymer chemistry, and biochemistry.
Its founding business areas were fibers and textiles, ...
photochemical production of ε-caprolactame.
*Photochemical production of artemisinin
Artemisinin () and its semisynthetic derivatives are a group of drugs used in the treatment of malaria due to ''Plasmodium falciparum''. It was discovered in 1972 by Tu Youyou, who shared the 2015 Nobel Prize in Physiology or Medicine for her dis ...
, anti-malaria drug.
* Photoalkylation, used for the light-induced addition of alkyl groups to molecules.
Organic photochemistry
Examples of photochemicalorganic reactions
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical r ...
are electrocyclic reaction In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement where the net result is one pi bond being converted into one sigma bond or vice versa. These reactions are usually categorized by the following criteria:
* React ...
s, radical reaction
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired electron, unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemical reaction, chemi ...
s, photoisomerization
In chemistry, photoisomerization is a form of isomerization induced by photoexcitation. Both reversible and irreversible photoisomerizations are known for photoswitchable compounds. The term "photoisomerization" usually, however, refers to a re ...
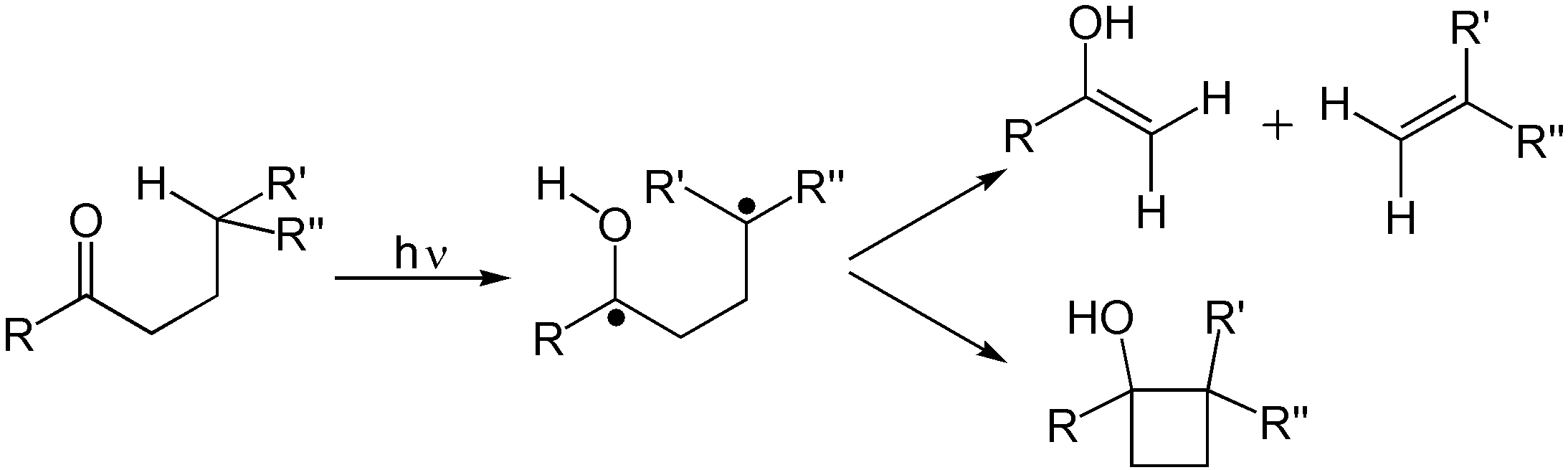
and Norrish reaction A Norrish reaction in organic chemistry is a photochemical reaction taking place with ketones and aldehydes. Such reactions are subdivided into Norrish type I reactions and Norrish type II reactions. The reaction is named after Ronald George Wreyfo ...
s.

Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s undergo many important reactions that proceed via a photon-induced π to π* transition. The first electronic excited state of an alkene lack the π-bond, so that rotation about the C-C bond is rapid and the molecule engages in reactions not observed thermally. These reactions include cis-trans isomerization, cycloaddition to other (ground state) alkene to give cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercial ...
derivatives. The cis-trans isomerization of a (poly)alkene is involved in retinal
Retinal (also known as retinaldehyde) is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision).
Some microorganisms use retin ...
, a component of the machinery of vision
Vision, Visions, or The Vision may refer to:
Perception Optical perception
* Visual perception, the sense of sight
* Visual system, the physical mechanism of eyesight
* Computer vision, a field dealing with how computers can be made to gain un ...
. The dimerization of alkenes is relevant to the photodamage of DNA, where thymine dimer
Pyrimidine dimers are molecular lesions formed from thymine or cytosine bases in DNA via photochemical reactions, commonly associated with direct DNA damage. Ultraviolet light (UV; particularly UVB) induces the formation of covalent linkages betwe ...
s are observed upon illuminating DNA to UV radiation. Such dimers interfere with transcription
Transcription refers to the process of converting sounds (voice, music etc.) into letters or musical notes, or producing a copy of something in another medium, including:
Genetics
* Transcription (biology), the copying of DNA into RNA, the fir ...
. The beneficial effects of sunlight are associated with the photochemically induced retro-cyclization (decyclization) reaction of ergosterol
Ergosterol (ergosta-5,7,22-trien-3β-ol) is a sterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergosterol, the ...
to give vitamin D
Vitamin D is a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, and many other biological effects. In humans, the most important compounds in this group are vitamin D3 (c ...
.
In the DeMayo reaction, an alkene reacts with a 1,3-diketone reacts via its enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene ( olefin) with a hydroxyl group attached to one end of the alkene double bond (). The te ...
to yield a 1,5-diketone. Still another common photochemical reaction is Howard Zimmerman
Howard E. Zimmerman (July 5, 1926 – February 12, 2012) was a professor of chemistry at the University of Wisconsin–Madison. He was elected to the National Academy of Sciences in 1980 and the recipient of the 1986 American Institute of Chemist ...
's di-π-methane rearrangement The di-π-methane rearrangement is a photochemical reaction of a molecular entity that contains two π-systems separated by a saturated carbon atom (a 1,4-diene or an allyl-substituted aromatic ring), to form an ene- (or aryl-) substituted cyclop ...
.
In an industrial application, about 100,000 tonnes of benzyl chloride
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.
Preparation
Benzyl chloride is prepared indust ...
are prepared annually by the gas-phase photochemical reaction of toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) at ...
with chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
. The light is absorbed by chlorine molecule, the low energy of this transition being indicated by the yellowish color of the gas. The photon induces homolysis of the Cl-Cl bond, and the resulting chlorine radical converts toluene to the benzyl radical:
:Cl2 + hν → 2 Cl·
:C6H5CH3 + Cl· → C6H5CH2· + HCl
:C6H5CH2· + Cl· → C6H5CH2Cl
Mercaptans
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
can be produced by photochemical addition of hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
(H2S) to alpha olefins
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
.
Inorganic and organometallic photochemistry
Coordination complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
es and organometallic compound
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
s are also photoreactive. These reactions can entail cis-trans isomerization. More commonly photoreactions result in dissociation of ligands, since the photon excites an electron on the metal to an orbital that is antibonding with respect to the ligands. Thus, metal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe ch ...
s that resist thermal substitution undergo decarbonylation upon irradiation with UV light. UV-irradiation of a THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
solution of molybdenum hexacarbonyl
Molybdenum hexacarbonyl (also called molybdenum carbonyl) is the chemical compound with the formula Mo(CO)6. This colorless solid, like its chromium and tungsten analogues, is noteworthy as a volatile, air-stable derivative of a metal in its zero ...
gives the THF complex, which is synthetically useful:
:Mo(CO)6 + THF → Mo(CO)5(THF) + CO
In a related reaction, photolysis of iron pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula . Under standard conditions Fe( CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to ...
affords diiron nonacarbonyl
Diiron nonacarbonyl is an organometallic compound with the formula Fe2(CO)9. This metal carbonyl is an important reagent in organometallic chemistry and of occasional use in organic synthesis. It is a more reactive source of Fe(0) than Fe(CO)5. ...
(see figure):
:2 Fe(CO)5 → Fe2(CO)9 + CO
Select photoreactive coordination complexes can undergo oxidation-reduction processes via single electron transfer. This electron transfer can occur within the inner
Interior may refer to:
Arts and media
* ''Interior'' (Degas) (also known as ''The Rape''), painting by Edgar Degas
* ''Interior'' (play), 1895 play by Belgian playwright Maurice Maeterlinck
* ''The Interior'' (novel), by Lisa See
* Interior de ...
or outer
{{Short pages monitor