|
Radical Polymerization
In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks ( repeat units). Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain. Free-radical polymerization is a key synthesis route for obtaining a wide variety of different polymers and materials composites. The relatively non-specific nature of free-radical chemical interactions makes this one of the most versatile forms of polymerization available and allows facile reactions of polymeric free-radical chain ends and other chemicals or substrates. In 2001, 40 billion of the 110 billion pounds of polymers produced in the United States were produced by free-radical polymerization. Free-radical polymerization is a type of chain-growth polyme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer Chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures of chemicals, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applicable through a wide range of other chemistry sub-disciplines like organic chemistry, analytical chemistry, and physical chemistry. Many materials have polymeric structures, from fully inorganic metals and ceramics to DNA and other biological molecules. However, polymer chemistry is typically related to synthetic and organic compositions. Synthetic polymers are ubiquitous in commercial materials and products in everyday use, such as plastics, and rubbers, and are major components of composite materials. Polymer chemistry can also be included in the broader fields of polymer science or even nanotechnology, both of which can be described as encompassing polymer physics and polymer engineering.Hans-Heinrich Moretto, Manfred Sc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical (chemistry)
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired electron, unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemical reaction, chemically reactive. Many radicals spontaneously dimer (chemistry), dimerize. Most organic radicals have short lifetimes. A notable example of a radical is the hydroxyl radical (HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and methylene radical, triplet carbene (꞉) which have two unpaired electrons. Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation, heat, electrical discharges, and electrolysis are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations. Radicals are important in combustion, atmospheric chemistry, polymerization, Plasma (ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Persulfate
A persulfate (sometimes known as peroxysulfate or peroxodisulfate) is a compound containing the anions or . The anion contains one peroxide group per sulfur center, whereas in , the peroxide group bridges the sulfur atoms. In both cases, sulfur adopts the normal tetrahedral geometry typical for the S(VI) oxidation state. These salts are strong oxidizers. Ions * Peroxomonosulfate ion, * Peroxydisulfate Acids * Peroxymonosulfuric acid (Caro's acid), H2SO5 * Peroxydisulfuric acid, H2S2O8 Example salts * Sodium peroxomonosulfate, Na2SO5 * Potassium peroxymonosulfate, KHSO5 * Sodium persulfate (sodium peroxydisulfate), Na2S2O8 * Ammonium persulfate Ammonium persulfate (APS) is the inorganic compound with the formula (NH4)2S2O8. It is a colourless (white) salt that is highly soluble in water, much more so than the related potassium salt. It is a strong oxidizing agent that is used as a catalys ... (ammonium peroxydisulfate), (NH4)2S2O8 * Potassium persulfate (potassium per ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prepolymer
In polymer chemistry, the term prepolymer or pre-polymer, refers to a monomer or system of monomers that have been reacted to an intermediate-molecular mass state. This material is capable of further polymerization by reactive groups to a fully cured, high-molecular-mass state. As such, mixtures of reactive polymers with un-reacted monomers may also be referred to as pre-polymers. The term "pre-polymer" and "polymer precursor" may be interchanged. Polyurethane and polyurea prepolymers In polyurethane chemistry, prepolymers and oligomers are frequently produced and then further formulated into CASE applications - Coatings, Adhesives, Sealants, and Elastomers. An isocyanate (usually a diisocyanate) is reacted with a polyol. All types of polyol may in theory be used to produce polyurethane prepolymers. These then find use in CASE applications. When polyurethane dispersions are synthesized, a prepolymer is first produced usually modified with DMPA. In polyurea prepolymer production ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinyl Group
In organic chemistry, a vinyl group (abbr. Vi; IUPAC name: ethenyl group) is a functional group with the formula . It is the ethylene (IUPAC name: ethene) molecule () with one fewer hydrogen atom. The name is also used for any compound containing that group, namely where R is any other group of atoms. An industrially important example is vinyl chloride, precursor to PVC, a plastic commonly known as ''vinyl''. Vinyl is one of the alkenyl functional groups. On a carbon skeleton, sp2-hybridized carbons or positions are often called vinylic. Allyls, acrylates and styrenics contain vinyl groups. (A styrenic crosslinker with two vinyl groups is called '' divinyl benzene''.) Vinyl polymers Vinyl groups can polymerize with the aid of a radical initiator or a catalyst, forming vinyl polymers. Vinyl polymers contain no vinyl groups. Instead they are saturated. The following table gives some examples of vinyl polymers. Reactivity Vinyl derivatives are alkenes. If act ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorbance
Absorbance is defined as "the logarithm of the ratio of incident to transmitted radiant power through a sample (excluding the effects on cell walls)". Alternatively, for samples which scatter light, absorbance may be defined as "the negative logarithm of one minus absorptance, as measured on a uniform sample". The term is used in many technical areas to quantify the results of an experimental measurement. While the term has its origin in quantifying the absorption of light, it is often entangled with quantification of light which is “lost” to a detector system through other mechanisms. What these uses of the term tend to have in common is that they refer to a logarithm of the ratio of a quantity of light incident on a sample or material to that which is detected after the light has interacted with the sample. The term absorption refers to the physical process of absorbing light, while absorbance does not always measure only absorption; it may measure attenuation (of tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Initiation - Photolysis
Initiation is a rite of passage marking entrance or acceptance into a group or society. It could also be a formal admission to adulthood in a community or one of its formal components. In an extended sense, it can also signify a transformation in which the initiate is 'reborn' into a new role. Examples of initiation ceremonies might include Christian baptism or confirmation, Jewish bar or bat mitzvah, acceptance into a fraternal organization, secret society or religious order, or graduation from school or recruit training. A person taking the initiation ceremony in traditional rites, such as those depicted in these pictures, is called an ''initiate''. See also rite of passage. Characteristics William Ian Miller notes the role of ritual humiliation in comic ordering and testing. Mircea Eliade discussed initiation as a principal religious act by classical or traditional societies. He defined initiation as "a basic change in existential condition", which liberates man from prof ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. Photodissociation is not limited to visible light. Any photon with sufficient energy can affect the chemical bonds of a chemical compound. Since a photon's energy is inversely proportional to its wavelength, electromagnetic radiations with the energy of visible light or higher, such as ultraviolet light, x-rays, and gamma rays can induce such reactions. Photolysis in photosynthesis Photolysis is part of the light-dependent reaction or light phase or photochemical phase or Hill reaction of photosynthesis. The general reaction of photosynthetic photolysis can be given in terms of photons as: :\ce + 2 \text \longrightarrow \ce The chemical nature of "A" depends on the type of organism. Purple sulfur bacteria oxidize hydrogen sulfide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Initiation - Thermal Decomp
Initiation is a rite of passage marking entrance or acceptance into a group or society. It could also be a formal admission to adulthood in a community or one of its formal components. In an extended sense, it can also signify a transformation in which the initiate is 'reborn' into a new role. Examples of initiation ceremonies might include Christian baptism or confirmation, Jewish bar or bat mitzvah, acceptance into a fraternal organization, secret society or religious order, or graduation from school or recruit training. A person taking the initiation ceremony in traditional rites, such as those depicted in these pictures, is called an ''initiate''. See also rite of passage. Characteristics William Ian Miller notes the role of ritual humiliation in comic ordering and testing. Mircea Eliade discussed initiation as a principal religious act by classical or traditional societies. He defined initiation as "a basic change in existential condition", which liberates man from prof ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
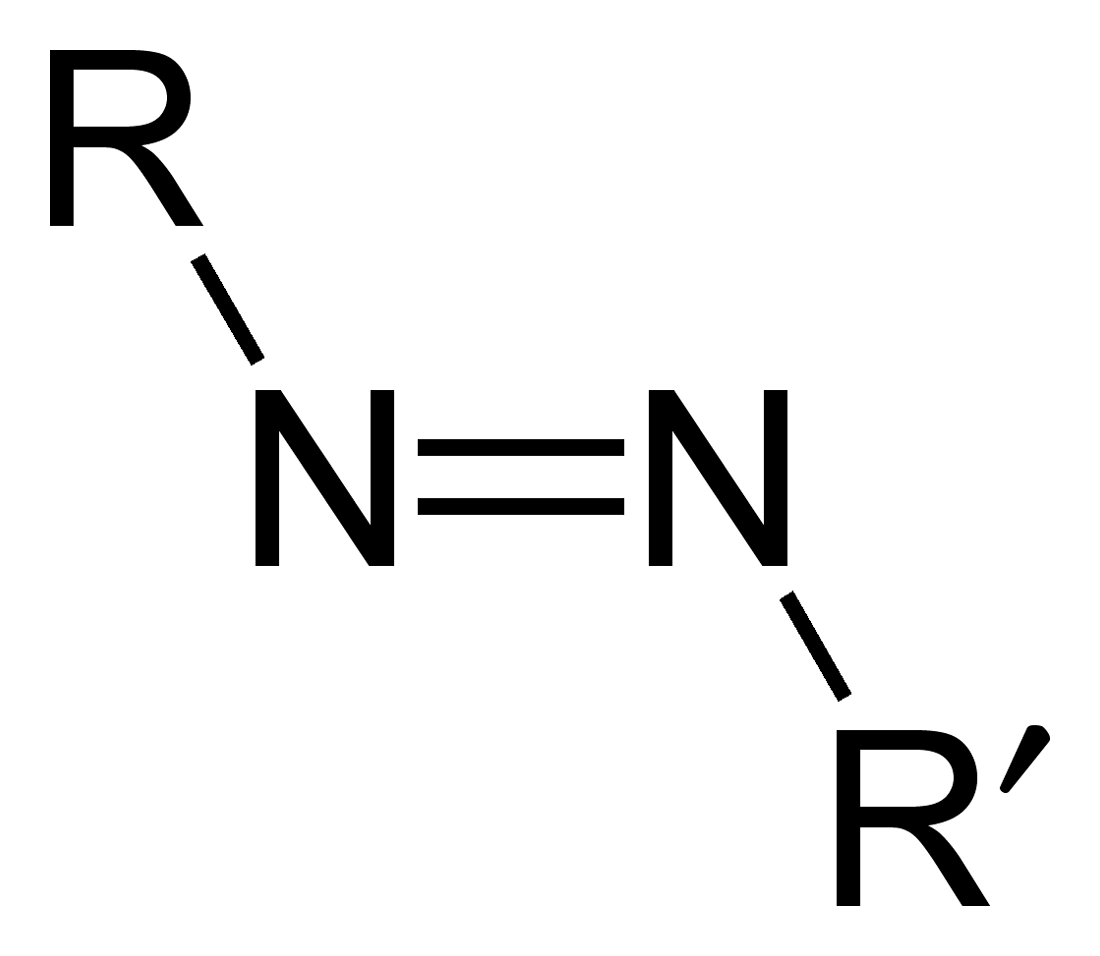
Azo Compounds
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the ''trans'' isomer, but upon illumination, converts to the ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating groups: :ArN2+ + Ar'H -> ArN=NAr' + H+ Since diazon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |






.jpg)