|
Organic Reactions
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic chemistry has a strong tradition of naming a specific reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Claisen Rearrangement Scheme
Claisen may refer to: *Rainer Ludwig Claisen, a German chemist **Claisen rearrangement, a reaction of a allyl vinyl ether to a γ,δ-unsaturated carbonyl **Claisen condensation, a reaction between esters and carbonyl compounds in the presence of a strong base **Ireland–Claisen rearrangement, a chemical reaction of an allylic ester with strong base **Claisen isatin synthesis Claisen may refer to: *Rainer Ludwig Claisen, a German chemist **Claisen rearrangement, a reaction of a allyl vinyl ether to a γ,δ-unsaturated carbonyl **Claisen condensation, a reaction between esters and carbonyl compounds in the presence of ... See also * 5243 Clasien, a minor planet {{Disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wöhler Synthesis
The Wöhler synthesis is the conversion of ammonium cyanate into urea. This chemical reaction was described in 1828 by Friedrich Wöhler. It is often cited as the starting point of modern organic chemistry. Although the Wöhler reaction concerns the conversion of ammonium cyanate, this salt appears only as an (unstable) intermediate. Wöhler demonstrated the reaction in his original publication with different sets of reactants: a combination of cyanic acid and ammonia, a combination of silver cyanate and ammonium chloride, a combination of lead cyanate and ammonia and finally from a combination of mercury cyanate and cyanatic ammonia (which is again cyanic acid with ammonia). Modified versions of the Wöhler synthesis The reaction can be demonstrated by starting with solutions of potassium cyanate and ammonium chloride which are mixed, heated and cooled again. An additional proof of the chemical transformation is obtained by adding a solution of oxalic acid which forms urea ox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Reaction
The aldol reaction is a means of forming carbon–carbon bonds in organic chemistry. Discovered independently by the Russian chemist Alexander Borodin in 1869 and by the French chemist Charles-Adolphe Wurtz in 1872, the reaction combines two carbonyl compounds (the original experiments used aldehydes) to form a new β-hydroxy carbonyl compound. These products are known as ''aldols'', from the ''ald''ehyde + alcoh''ol'', a structural motif seen in many of the products. Aldol structural units are found in many important molecules, whether naturally occurring or synthetic. For example, the aldol reaction has been used in the large-scale production of the commodity chemical pentaerythritol and the synthesis of the heart disease drug Lipitor (atorvastatin, calcium salt). The aldol reaction unites two relatively simple molecules into a more complex one. Increased complexity arises because up to two new stereogenic centers (on the Alpha carbon, α- and β-carbon of the aldol adduct, mar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
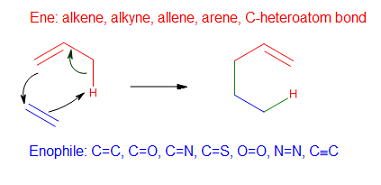
Ene Reaction
In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position. This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Many useful Lewis acid-catalyzed ene reactions have been also developed, which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products. Ene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CBS Reduction
CBS Broadcasting Inc., commonly shortened to CBS, the abbreviation of its former legal name Columbia Broadcasting System, is an American commercial broadcast television and radio network serving as the flagship property of the CBS Entertainment Group division of Paramount Global. Its headquarters is at the CBS Building in New York City. It has major production facilities and operations at the CBS Broadcast Center and the headquarters of owner Paramount Global at One Astor Plaza (both also in that city) and Television City and the CBS Studio Center in Los Angeles. It is also sometimes referred to as the Eye Network in reference to the company's trademark symbol which has been in use since 1951. It has also been called the Tiffany Network which alludes to the perceived high quality of its programming during the tenure of William S. Paley. It can also refer to some of CBS's first demonstrations of color television, which were held in the former Tiffany and Company Building in New ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bingel Reaction
The Bingel reaction in fullerene chemistry is a fullerene cyclopropanation reaction to a methanofullerene first discovered by C. Bingel in 1993 with the bromo derivative of diethyl malonate in the presence of a base such as sodium hydride or DBU. The preferred double bonds for this reaction on the fullerene surface are the shorter bonds at the junctions of two hexagons (6-6 bonds) and the driving force is relief of steric strain. The reaction is of importance in the field of chemistry because it allows the introduction of useful extensions to the fullerene sphere. These extensions alter their properties, for instance solubility and electrochemical behavior, and therefore widen the range of potential technical applications. Reaction mechanism The reaction mechanism for this reaction is as follows: a base abstracts the acidic malonate proton generating a carbanion or enolate which reacts with the electron deficient fullerene double bond in a nucleophilic addition. This in turn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Claisen Rearrangement
The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a ,3sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, driven by exergonically favored carbonyl CO bond formation (ΔΔHf = -327kcalmol−1). Mechanism The Claisen rearrangement is an exothermic, concerted (bond cleavage and recombination) pericyclic reaction. Woodward–Hoffmann rules show a suprafacial, stereospecific reaction pathway. The kinetics are of the first order and the whole transformation proceeds through a highly ordered cyclic transition state and is intramolecular. Crossover experiments eliminate the possibility of the rearrangement occurring via an intermolecular reaction mechanism and are consistent with an intramolecular process. There are substantial solvent effects observed in the Claisen rearrangement, where polar solvents tend to accelerate the reaction to a greater e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Named Reaction
A name reaction is a chemical reaction named after its discoverers or developers. Among the tens of thousands of organic reactions that are known, hundreds of such reactions are well-known enough to be named after people. Well-known examples include the Grignard reaction, the Sabatier reaction, the Wittig reaction, the Claisen condensation, the Friedel-Crafts acylation, and the Diels-Alder reaction. Books have been published devoted exclusively to name reactions;Alfred Hassner, C. Stumer. ''Organic syntheses based on name reactions''. Elsevier, 2002. Li, Jie Jack. ''Name Reactions: A Collection of Detailed Reaction Mechanisms''. Springer, 2003. the Merck Index, a chemical encyclopedia, also includes an appendix on name reactions. As organic chemistry developed during the 20th century, chemists started associating synthetically useful reactions with the names of the discoverers or developers; in many cases, the name is merely a mnemonic. Some cases of reactions that were not reall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Organic Reactions
Well-known reactions and reagents in organic chemistry include 0-9 * 1,2-Wittig rearrangement * 1,3-Dipolar cycloaddition *2,3-Wittig rearrangement A * Abramovitch–Shapiro tryptamine synthesis *Acetalisation * Acetoacetic ester condensation *Achmatowicz reaction *Acylation *Acyloin condensation *Adams' catalyst * Adams decarboxylation * Adkins catalyst * Adkins–Peterson reaction * Akabori amino acid reaction *Alcohol oxidation * Alder ene reaction * Alder–Stein rules * Aldol addition *Aldol condensation *Algar–Flynn–Oyamada reaction *Alkylimino-de-oxo-bisubstitution *Alkyne trimerisation * Alkyne zipper reaction *Allan–Robinson reaction *Allylic rearrangement *Amadori rearrangement * Amine alkylation * Angeli–Rimini reaction *Andrussov oxidation *Appel reaction *Arbuzov reaction, Arbusow reaction *Arens–Van Dorp synthesis, Isler modification *Aromatic nitration * Arndt–Eistert synthesis * Aston–Greenburg rearrangement *Auwers synthesis *Aza-Cope rearrangeme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Olefin Metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creates fewer undesired by-products and hazardous wastes than alternative organic reactions. For their elucidation of the reaction mechanism and their discovery of a variety of highly active catalysts, Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock were collectively awarded the 2005 Nobel Prize in Chemistry. Catalysts The reaction requires metal catalysts. Most commercially important processes employ heterogeneous catalysts. The heterogeneous catalysts are often prepared by in-situ activation of a metal halides (MClx) using organoaluminium or organotin compounds, e.g. combining MClx–EtAlCl2. A typical catalyst support is alumina. Commercial catalysts are often based on molybdenum and ruthenium. Well-defined organometallic co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wittig Reaction
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most often, the Wittig reaction is used to introduce a methylene group using methylenetriphenylphosphorane (Ph3P=CH2). Using this reagent, even a sterically hindered ketone such as camphor can be converted to its methylene derivative. Stereochemistry For the reaction with aldehydes, the double bond geometry is readily predicted based on the nature of the ylide. With unstabilised ylides (R3 = alkyl) this results in (''Z'')-alkene product with moderate to high selectivity. With stabilized ylides (R3 = ester or ketone), the (''E'')-alkene is formed with high selectivity. The (''E'')/(''Z'') selectivity is often poor with semistabilized ylides (R3 = aryl). To obtain the (''E'')-alkene for unstabilized ylides, the Schlosser modification of the W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |