Phosphinidene Singlet Triplet on:
[Wikipedia]
[Google]
[Amazon]
 Phosphinidenes (IUPAC: phosphanylidenes, formerly phosphinediyls) are low-valent phosphorus compounds analogous to
Phosphinidenes (IUPAC: phosphanylidenes, formerly phosphinediyls) are low-valent phosphorus compounds analogous to
 The ground state in the parent phosphinidene (PH) is a triplet that is 22 kcal/mol more stable than the lowest singlet state. This singlet-triplet energy gap is considerably larger than that of the simplest carbene methylene (9 kcal/mol).
Ab initio calculations from Nguyen et al. found that alkyl- and silyl-substituted phosphinidenes have triplet ground states, possibly in-part due to a negative hyperconjugation effect that stabilizes the triplet more than the singlet. Substituents containing lone pairs (e.g. -NX2, -OX, -PX2 ,-SX) were found to stabilize the singlet state, presumably by π-donation into an empty phosphorus 3p orbital; in most of these cases, the energies of the lowest singlet and triplet states were close to degenerate. A singlet ground state could be induced in amino- and phosphino-phosphinidenes by introducing bulky β-substituents, which are thought to destabilize the triplet state by distorting the pyramidal geometry through increased nuclear repulsion.
The ground state in the parent phosphinidene (PH) is a triplet that is 22 kcal/mol more stable than the lowest singlet state. This singlet-triplet energy gap is considerably larger than that of the simplest carbene methylene (9 kcal/mol).
Ab initio calculations from Nguyen et al. found that alkyl- and silyl-substituted phosphinidenes have triplet ground states, possibly in-part due to a negative hyperconjugation effect that stabilizes the triplet more than the singlet. Substituents containing lone pairs (e.g. -NX2, -OX, -PX2 ,-SX) were found to stabilize the singlet state, presumably by π-donation into an empty phosphorus 3p orbital; in most of these cases, the energies of the lowest singlet and triplet states were close to degenerate. A singlet ground state could be induced in amino- and phosphino-phosphinidenes by introducing bulky β-substituents, which are thought to destabilize the triplet state by distorting the pyramidal geometry through increased nuclear repulsion.
 Density functional theory and Natural bond orbital (NBO) calculations were used to gain insight into the structure and bonding of these phosphino-phosphinidenes. DFT calculations at the M06-2X/Def2-SVP level of theory on the phospino-phosphinidene with bulky 2,6-bis -tert-butylphenyl)methyl4-methylphenyl groups suggest that the tri-coordinated phosphorus atom exists in a planar environment. Calculations at the ''M06-2X/def2-TZVPP//M06-2X/def2-SVP'' level of theory were applied to a simplified model compound with diisopropylphenyl (Dipp) groups so as to reduce the computational cost for detailed NBO analysis. Inspection of the outputted wavefunctions shows that the
Density functional theory and Natural bond orbital (NBO) calculations were used to gain insight into the structure and bonding of these phosphino-phosphinidenes. DFT calculations at the M06-2X/Def2-SVP level of theory on the phospino-phosphinidene with bulky 2,6-bis -tert-butylphenyl)methyl4-methylphenyl groups suggest that the tri-coordinated phosphorus atom exists in a planar environment. Calculations at the ''M06-2X/def2-TZVPP//M06-2X/def2-SVP'' level of theory were applied to a simplified model compound with diisopropylphenyl (Dipp) groups so as to reduce the computational cost for detailed NBO analysis. Inspection of the outputted wavefunctions shows that the  Despite the negative charge on the terminal phosphorus atom, subsequent studies have shown that this particular phosphinidene is electrophilic at the phosphinidene center. This phosphino-phosphinidene reacts with a number of nucleophiles (CO, isocyanides, carbenes, phosphines, etc.) to form phosphinidene-nucleophile adducts Upon nucleophilic addition, the tri-coordinated phosphorus atom becomes non-planar, and it is postulated that the driving force of the reaction is provided by the instability of the phosphinidene's planar geometry.
Despite the negative charge on the terminal phosphorus atom, subsequent studies have shown that this particular phosphinidene is electrophilic at the phosphinidene center. This phosphino-phosphinidene reacts with a number of nucleophiles (CO, isocyanides, carbenes, phosphines, etc.) to form phosphinidene-nucleophile adducts Upon nucleophilic addition, the tri-coordinated phosphorus atom becomes non-planar, and it is postulated that the driving force of the reaction is provided by the instability of the phosphinidene's planar geometry.

 In 1989, Fritz et al. synthesized the phospha-Wittig species shown to the right. Phospha-Wittig compounds can be viewed as a phosphinidene stabilized by a phosphine. These compounds have been given the label of "phospha-Wittig" as they have two dominant resonance structures (a neutral form and a
In 1989, Fritz et al. synthesized the phospha-Wittig species shown to the right. Phospha-Wittig compounds can be viewed as a phosphinidene stabilized by a phosphine. These compounds have been given the label of "phospha-Wittig" as they have two dominant resonance structures (a neutral form and a 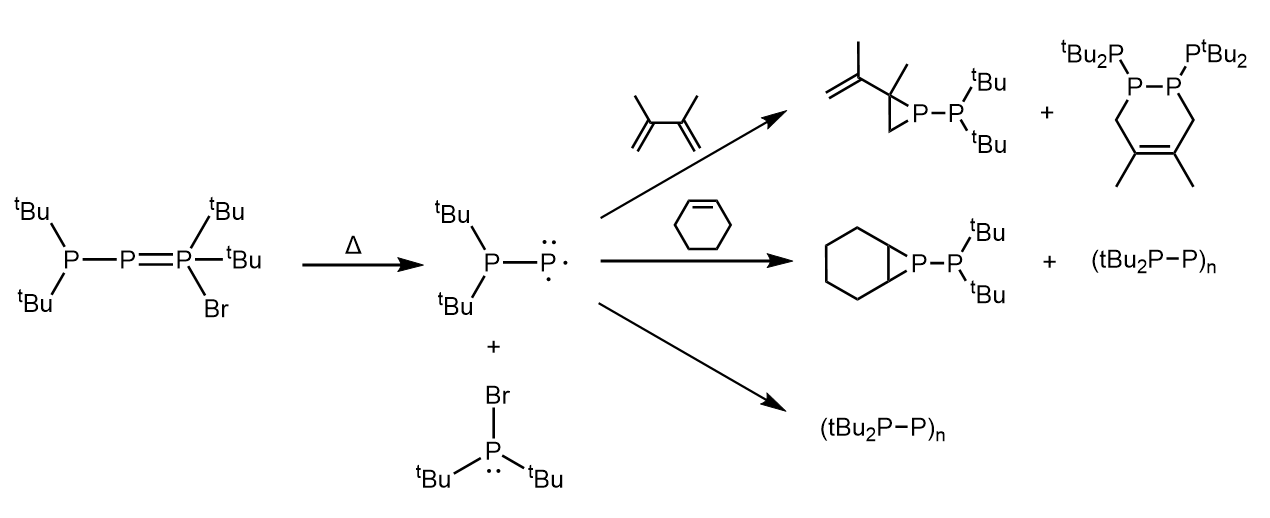
 Lappert and coworkers reported the first synthesis of a stable terminal phosphinidene complex: lithium metallocene hydrides p2MHLisub>4 of Mo and W were reacted with aryl-dichlorophosphines RPCl2 to yield Cp2M=P-R, which were able to be characterized by single crystal X-ray diffraction.
Lappert and coworkers reported the first synthesis of a stable terminal phosphinidene complex: lithium metallocene hydrides p2MHLisub>4 of Mo and W were reacted with aryl-dichlorophosphines RPCl2 to yield Cp2M=P-R, which were able to be characterized by single crystal X-ray diffraction.
 More common than complexes of terminal phosphinidene ligands are
More common than complexes of terminal phosphinidene ligands are
 Molecular beam mass spectrometry has enabled the detection of the evolution of amino-phosphinidene fragments from a number of alkylamide derivatives (e.g. Me2NP+ and Me2NPH+ from Me2NPA) in the gas-phase at elevated temperatures.
Molecular beam mass spectrometry has enabled the detection of the evolution of amino-phosphinidene fragments from a number of alkylamide derivatives (e.g. Me2NP+ and Me2NPH+ from Me2NPA) in the gas-phase at elevated temperatures.
 Phosphinidenes (IUPAC: phosphanylidenes, formerly phosphinediyls) are low-valent phosphorus compounds analogous to
Phosphinidenes (IUPAC: phosphanylidenes, formerly phosphinediyls) are low-valent phosphorus compounds analogous to carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
s and nitrene
In chemistry, a nitrene or imene () is the nitrogen analogue of a carbene. The nitrogen atom is uncharged and univalent, so it has only 6 electrons in its valence level—two covalent bonded and four non-bonded electrons. It is therefore cons ...
s, having the general structure RP. The "free" form of these compounds is conventionally described as having a singly-coordinated phosphorus atom containing only 6 electrons in its valence level. Most phosphinidenes are highly reactive and short-lived, thereby complicating empirical studies on their chemical properties. In the last few decades, several strategies have been employed to stabilize phosphinidenes (e.g. π-donation, steric protection, transition metal complexation), and researchers have developed a number of reagents and systems that can generate and transfer phosphinidenes as reactive intermediates in the synthesis of various organophosphorus compounds.Electronic Structure
Like carbenes, phosphinidenes can exist in either a singlet state or triplet state, with the triplet state typically being more stable. The stability of these states and their relative energy difference (the singlet-triplet energy gap) is dependent on the substituents. The ground state in the parent phosphinidene (PH) is a triplet that is 22 kcal/mol more stable than the lowest singlet state. This singlet-triplet energy gap is considerably larger than that of the simplest carbene methylene (9 kcal/mol).
Ab initio calculations from Nguyen et al. found that alkyl- and silyl-substituted phosphinidenes have triplet ground states, possibly in-part due to a negative hyperconjugation effect that stabilizes the triplet more than the singlet. Substituents containing lone pairs (e.g. -NX2, -OX, -PX2 ,-SX) were found to stabilize the singlet state, presumably by π-donation into an empty phosphorus 3p orbital; in most of these cases, the energies of the lowest singlet and triplet states were close to degenerate. A singlet ground state could be induced in amino- and phosphino-phosphinidenes by introducing bulky β-substituents, which are thought to destabilize the triplet state by distorting the pyramidal geometry through increased nuclear repulsion.
The ground state in the parent phosphinidene (PH) is a triplet that is 22 kcal/mol more stable than the lowest singlet state. This singlet-triplet energy gap is considerably larger than that of the simplest carbene methylene (9 kcal/mol).
Ab initio calculations from Nguyen et al. found that alkyl- and silyl-substituted phosphinidenes have triplet ground states, possibly in-part due to a negative hyperconjugation effect that stabilizes the triplet more than the singlet. Substituents containing lone pairs (e.g. -NX2, -OX, -PX2 ,-SX) were found to stabilize the singlet state, presumably by π-donation into an empty phosphorus 3p orbital; in most of these cases, the energies of the lowest singlet and triplet states were close to degenerate. A singlet ground state could be induced in amino- and phosphino-phosphinidenes by introducing bulky β-substituents, which are thought to destabilize the triplet state by distorting the pyramidal geometry through increased nuclear repulsion.
Stable Monomeric Phosphino-Phosphinidene
Bertrand
Bertrand may refer to:
Places
* Bertrand, Missouri, US
* Bertrand, Nebraska, US
* Bertrand, New Brunswick, Canada
* Bertrand Township, Michigan, US
* Bertrand, Michigan
* Bertrand, Virginia, US
* Bertrand Creek, state of Washington
* Saint-Bert ...
and coworkers synthesized a stable singlet phosphino-phosphinidene compound using extremely bulky substituents. Hitherto, there had been no free singlet phosphinidenes that were characterized by spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wa ...
. The authors prepared a chlorodiazaphospholidine with bulky (2,6-bis 4-tert-butylphenyl)methyl4-methylphenyl) groups, and then synthesized the corresponding phosphaketene. Subsequent photolytic decarbonylation of the phosphaketene produced the phosphino-phosphinidene product as a yellow-orange solid that is stable at room temperature but decomposes immediately in the presence of air and moisture. 31P NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fiel ...
shows assigned product peaks at 80.2 and -200.4 ppm, with a J-coupling
In nuclear chemistry and nuclear physics, ''J''-couplings (also called spin-spin coupling or indirect dipole–dipole coupling) are mediated through chemical bonds connecting two spins. It is an indirect interaction between two nuclear spins that a ...
constant of JPP = 883.7 Hz. The very high P-P coupling constant is indicative of P-P multiple bond character. The air/water sensitivity and high solubility of this compound prevented characterization by X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
.  Density functional theory and Natural bond orbital (NBO) calculations were used to gain insight into the structure and bonding of these phosphino-phosphinidenes. DFT calculations at the M06-2X/Def2-SVP level of theory on the phospino-phosphinidene with bulky 2,6-bis -tert-butylphenyl)methyl4-methylphenyl groups suggest that the tri-coordinated phosphorus atom exists in a planar environment. Calculations at the ''M06-2X/def2-TZVPP//M06-2X/def2-SVP'' level of theory were applied to a simplified model compound with diisopropylphenyl (Dipp) groups so as to reduce the computational cost for detailed NBO analysis. Inspection of the outputted wavefunctions shows that the
Density functional theory and Natural bond orbital (NBO) calculations were used to gain insight into the structure and bonding of these phosphino-phosphinidenes. DFT calculations at the M06-2X/Def2-SVP level of theory on the phospino-phosphinidene with bulky 2,6-bis -tert-butylphenyl)methyl4-methylphenyl groups suggest that the tri-coordinated phosphorus atom exists in a planar environment. Calculations at the ''M06-2X/def2-TZVPP//M06-2X/def2-SVP'' level of theory were applied to a simplified model compound with diisopropylphenyl (Dipp) groups so as to reduce the computational cost for detailed NBO analysis. Inspection of the outputted wavefunctions shows that the HOMO
''Homo'' () is the genus that emerged in the (otherwise extinct) genus ''Australopithecus'' that encompasses the extant species ''Homo sapiens'' ( modern humans), plus several extinct species classified as either ancestral to or closely relate ...
and HOMO-1 are P-P π-bonding orbitals and the LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
is a P-P π*-antibonding orbital. Further evidence of multiple bond character between the phosphorus atoms was provided by natural resonance theory and a large Wiberg bond index (P1-P2: 2.34). Natural population analysis assigned a negative partial charge to the terminal phosphorus atom (-0.34 q) and a positive charge to the tri-coordinated phosphorus atom (1.16 q).
 Despite the negative charge on the terminal phosphorus atom, subsequent studies have shown that this particular phosphinidene is electrophilic at the phosphinidene center. This phosphino-phosphinidene reacts with a number of nucleophiles (CO, isocyanides, carbenes, phosphines, etc.) to form phosphinidene-nucleophile adducts Upon nucleophilic addition, the tri-coordinated phosphorus atom becomes non-planar, and it is postulated that the driving force of the reaction is provided by the instability of the phosphinidene's planar geometry.
Despite the negative charge on the terminal phosphorus atom, subsequent studies have shown that this particular phosphinidene is electrophilic at the phosphinidene center. This phosphino-phosphinidene reacts with a number of nucleophiles (CO, isocyanides, carbenes, phosphines, etc.) to form phosphinidene-nucleophile adducts Upon nucleophilic addition, the tri-coordinated phosphorus atom becomes non-planar, and it is postulated that the driving force of the reaction is provided by the instability of the phosphinidene's planar geometry.

Phospha-Wittig Fragmentation
 In 1989, Fritz et al. synthesized the phospha-Wittig species shown to the right. Phospha-Wittig compounds can be viewed as a phosphinidene stabilized by a phosphine. These compounds have been given the label of "phospha-Wittig" as they have two dominant resonance structures (a neutral form and a
In 1989, Fritz et al. synthesized the phospha-Wittig species shown to the right. Phospha-Wittig compounds can be viewed as a phosphinidene stabilized by a phosphine. These compounds have been given the label of "phospha-Wittig" as they have two dominant resonance structures (a neutral form and a zwitterionic
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively- and negatively-charged functional groups.
: With amino acids, for example, in solution a chemical equilibrium wil ...
form) that are analogous to those of the phosphonium ylides that are used in the Wittig reaction.
Fritz et al. found that this particular phospha-Wittig reagent thermally decomposes at 20 °C to give tBu2PBr, LiBr, and cyclophosphanes. The authors proposed that the singlet phosphino-phosphinidene tBu2PP was formed as an intermediate in this reaction. Further evidence for this was provided by trapping experiments, where the thermal decomposition of the phospha-Wittig reagent in the presence of 3,4,-dimethyl-1,3-butadiene and cyclohexene gave rise to the products shown in the figure below. 
Phosphinidene complexes
Terminal transition-metal-complexed phosphinidenes LnM=P-R are phosphorus analogs oftransition metal carbene complexes
Transition or transitional may refer to:
Mathematics, science, and technology Biology
* Transition (genetics), a point mutation that changes a purine nucleotide to another purine (A ↔ G) or a pyrimidine nucleotide to another pyrimidine (C ↔ ...
where L is a spectator ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
. The first terminal phosphinidene complex was reported by Marinetti et al., who observed the formation of the transient species OC)5M=P-Phduring the fragmentation of 7-phosphanorbornadiene molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
and tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolat ...
complexes inside a mass spectrometer. Soon after, they discovered that these 7-phosphanorbornadiene complexes could be used to transfer the phosphinidene complex OC)5M=P-Rto various unsaturated substrates.
 Lappert and coworkers reported the first synthesis of a stable terminal phosphinidene complex: lithium metallocene hydrides p2MHLisub>4 of Mo and W were reacted with aryl-dichlorophosphines RPCl2 to yield Cp2M=P-R, which were able to be characterized by single crystal X-ray diffraction.
Lappert and coworkers reported the first synthesis of a stable terminal phosphinidene complex: lithium metallocene hydrides p2MHLisub>4 of Mo and W were reacted with aryl-dichlorophosphines RPCl2 to yield Cp2M=P-R, which were able to be characterized by single crystal X-ray diffraction.
 More common than complexes of terminal phosphinidene ligands are
More common than complexes of terminal phosphinidene ligands are cluster compound
In chemistry, an atom cluster (or simply cluster) is an ensemble of bound atoms or molecules that is intermediate in size between a simple molecule and a nanoparticle; that is, up to a few nanometers (nm) in diameter. The term ''microcluster'' ...
s wherein the phosphinidene is a triply and less commonly doubly bridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually r ...
. One example is the ter-butylphosphinidene complex (t-BuP)Fe3(CO)10.
Dibenzo-7-phosphanorbornadiene derivatives
A class of RPA (A = anthracene) compounds were developed and explored byCummins
Cummins Inc. is an American multinational corporation that designs, manufactures, and distributes engines, filtration, and power generation products. Cummins also services engines and related equipment, including fuel systems, controls, air ...
and coworkers.
Treatment of a bulky phosphine chloride (RPCl2) with magnesium anthracene
Magnesium anthracene is an organomagnesium compound that is almost invariably isolated as its adduct with three tetrahydrofuran (thf) ligands. With the formula Mg(C14H10)(thf)3, this air- and water-sensitive orange solid is obtained by heating a ...
affords a dibenzo-7-phosphanorbornadiene compound (RPA). Under thermal conditions, the RPA compound (R = NiPr2) decomposes to yield anthracene; kinetic experiments found this decomposition to be first-order. It was hypothesized that the amino-phosphinidene iPr2NP is formed as a transient intermediate species, and this was corroborated by an experiment where 1,3-cyclohexadiene
Cyclohexa-1,3-diene is an organic compound with the formula (C2H4)(CH)4. It is a colorless, flammable liquid. Its refractive index is 1.475 (20 °C, D). A naturally occurring derivative of 1,3-cyclohexadiene is terpinene, a component of pine oi ...
was used as a trapping agent, forming ''anti''-iPr2NP(C6H8).
 Molecular beam mass spectrometry has enabled the detection of the evolution of amino-phosphinidene fragments from a number of alkylamide derivatives (e.g. Me2NP+ and Me2NPH+ from Me2NPA) in the gas-phase at elevated temperatures.
Molecular beam mass spectrometry has enabled the detection of the evolution of amino-phosphinidene fragments from a number of alkylamide derivatives (e.g. Me2NP+ and Me2NPH+ from Me2NPA) in the gas-phase at elevated temperatures.See also
*Carbene analog Carbene analogs in chemistry are carbenes with the carbon atom replaced by another chemical element. Just as regular carbenes they appear in chemical reactions as reactive intermediates and with special precautions they can be stabilized and isolate ...
*Phosphorus compounds
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
References
{{Reflist Reactive intermediates Organophosphorus compounds