|
Guy Bertrand (chemist)
Guy Bertrand, born on July 17, 1952 at Limoges is a chemistry professor at the University of California, San Diego. Bertrand obtained his B.Sc. from the University of Montpellier in 1975 and his Ph.D. from the Paul Sabatier University, Toulouse, in 1979. He was a postdoctoral researcher at Sanofi Research, France, in 1981. Guy Bertrand's faculty homepageat UC San Diego. Accessed on 2013-1-22. The research interests of Bertrand and his co-workers lie mainly in the chemistry of with main group elements from group 13 to 16, at the border between organic, organometallic and inorganic chemistry; especially their use in stabilizing carbenes, nitrenes, phosphinidenes, radicals and biradicals, 1,3-dipoles, anti-aromatic heterocycles, and more. He has directed the synthesis of some original persistent carbenes, including bis(diisopropylamino)cyclopropenylidene, the first example of a carbene with all-carbon environment that is stable at room-temperature.V. Lavallo, Y. Canac, B. Donna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many indus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
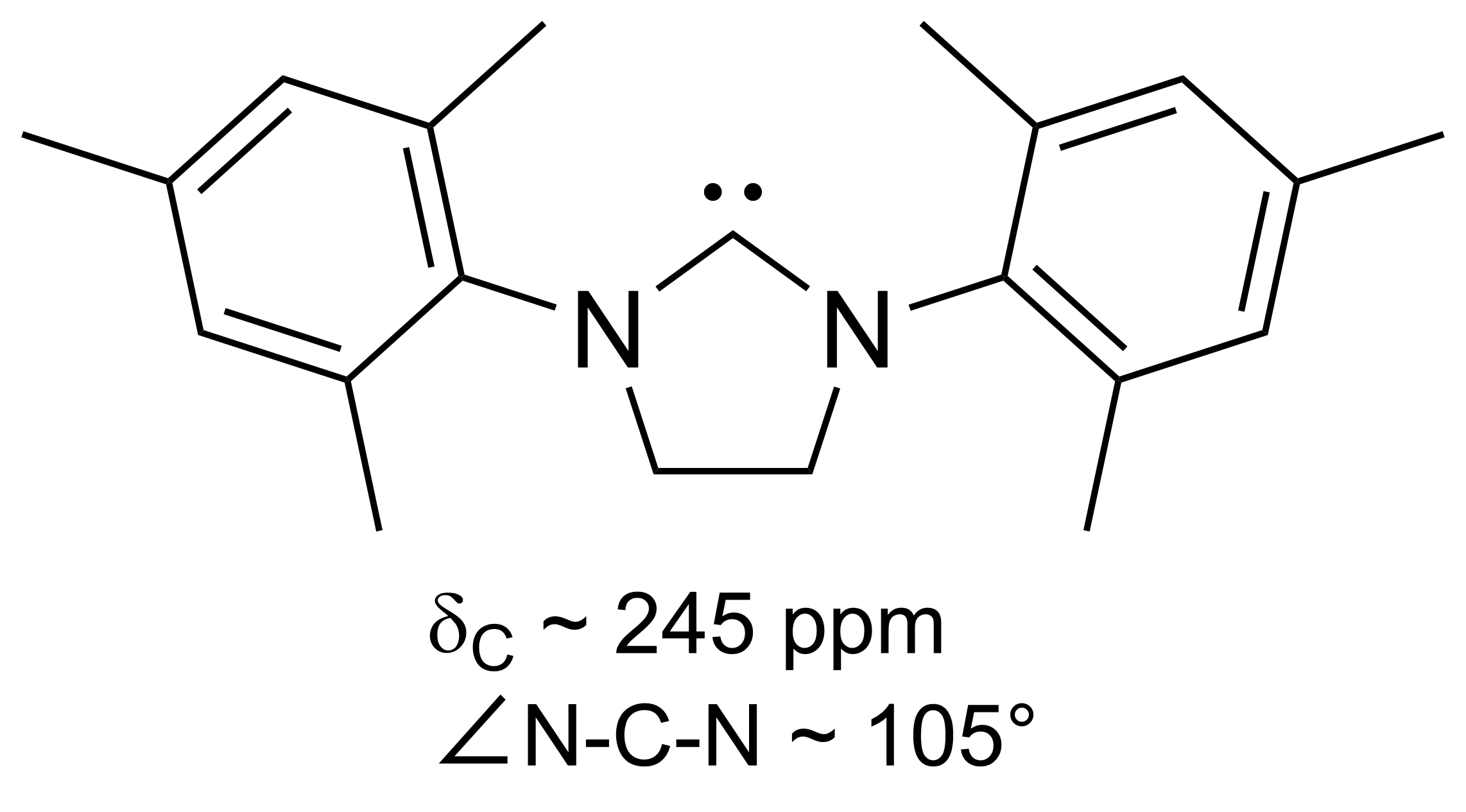
Cyclic Alkyl Amino Carbenes
In chemistry, cyclic(alkyl)(amino)carbenes (CAACs) are a family of stable singlet carbene ligands developed by Prof. Guy Bertrand anhis groupin 2005 at UC Riverside (now at UC San Diego). In marked contrast with the popular N-heterocyclic carbenes ( NHC) which possess two "amino" substituents adjacent to the "carbene" center, CAACs possess one "amino" substituent and an sp3 carbon atom "alkyl". This specific configuration makes the CAACs very good σ-donors (higher HOMO) and π-acceptors (lower LUMO) when compared to NHCs. Moreover the reduced heteroatom stabilization of the carbene center in CAACs versus NHCs also gives rise to a smaller ΔEST (48.3 vs 72.7 kcal mol-1). Synthesis The original preparation of CAACs precursors (Route 1) begins with condensation of 2,6-diisopropylaniline and 2-methylpropanal. Deprotonation of this aldimine with lithium diisopropylamide gives an aza-allyl anion, which ring opens 1,2-epoxy-2-methylpro-pane. The resulting lithium alkoxide is th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steric Effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindrance between adjacent groups can also affect torsional ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesoionic Carbene
In chemistry, mesoionic carbenes (MICs) are a type of reactive intermediate that are related to N-heterocyclic carbenes (NHCs); thus, MICs are also referred to as abnormal N-heterocyclic carbenes (aNHCs) or remote N-heterocyclic carbenes (rNHCs). Unlike simple NHCs, the canonical resonance structures of these carbenes are mesoionic: an MIC cannot be drawn without adding additional charges to some of the atoms. A variety of free carbenes can be isolated and are stable at room temperature. Other free carbenes are not stable and are susceptible to intermolecular decomposition pathways. MICs do not dimerize according to Wanzlick equilibrium as do normal NHCs. This results in relaxed steric requirements for mesoionic carbenes as compared to NHCs.G. Guisado-Barrios, J. Bouffard, B. Donnadieu, G. Bertrand. ''Angew. Chem., Int. Ed''. 2010, 49, 4759-4762.D. Martin, M. Melaimi, M. Soleilhavoup, G. Bertrand. ''Organometallics''. 2011, 30, 5304-5313.G. Ung, D. Mendoza-Espinosa, J. B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropenylidene
Cyclopropenylidene, or ''c''-C3H2, is a partially aromatic molecule belonging to a highly reactive class of organic molecules known as carbenes. On Earth, cyclopropenylidene is only seen in the laboratory due to its reactivity. However, cyclopropenylidene is found in significant concentrations in the interstellar medium (ISM) and on Saturn's moon Titan. Its C2v symmetric isomer, propadienylidene (CCCH2) is also found in the ISM, but with abundances about an order of magnitude lower. A third C2 symmetric isomer, propargylene (HCCCH), has not yet been detected in the ISM, most likely due to its low dipole moment. History The astronomical detection of ''c''-C3H2 was first confirmed in 1985.P. Thaddeus, J. M. Vrtilek, and C. A. Gottlieb "Laboratory and Astronomical Identification of Cyclopropenylidene, C3H2." ''Astrophys. J.'' 299 L63 (1985) Four years earlier, several ambiguous lines had been observed in the radio region of spectra taken of the ISM, but the observed lines we ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Persistent Carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole. Traditionally carbenes are viewed as so reactive that were only studied indirectly, such as by trapping reactions. This situation has changed dramatically with the emergence of persistent carbenes. Although they are fairly reactive substances, undergoing dimerization, many can be isolated as pure substances. Persistent carbenes tend to exist in the singlet. Their stability is only partly due to steric hindrance by bulky groups. Some singlet carbenes are thermodynamically stable and can be iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-heterocyclic Carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole. Traditionally carbenes are viewed as so reactive that were only studied indirectly, such as by trapping reactions. This situation has changed dramatically with the emergence of persistent carbenes. Although they are fairly reactive substances, undergoing dimerization, many can be isolated as pure substances. Persistent carbenes tend to exist in the singlet. Their stability is only partly due to steric hindrance by bulky groups. Some singlet carbenes are thermodynamically stable and can be iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term " metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are repres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Chemistry
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture. Key concepts Many inorganic compounds are ionic compounds, consisting of cations and anions joined by ionic bonding. Examples of salts (which are ionic compounds) are magnesium chloride MgCl2, which consists of magnesium cations Mg2+ and chloride anions Cl−; or sodium oxide Na2O, which consists of sodium cations Na+ and oxide anions O2−. In any salt, the proportions of the ions are such that the electric charges cancel out, so that the bulk compound is e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)