Cyclic Alkyl Amino Carbenes on:
[Wikipedia]
[Google]
[Amazon]
 In
In
his group
in 2005 at UC Riverside (now at UC San Diego). In marked contrast with the popular N-heterocyclic carbenes ( NHC) which possess two "amino" substituents adjacent to the "carbene" center, CAACs possess one "amino" substituent and an sp3 carbon atom "alkyl". This specific configuration makes the CAACs very good σ-donors (higher HOMO) and π-acceptors (lower LUMO) when compared to NHCs. Moreover the reduced heteroatom stabilization of the carbene center in CAACs versus NHCs also gives rise to a smaller ΔEST (48.3 vs 72.7 kcal mol-1).
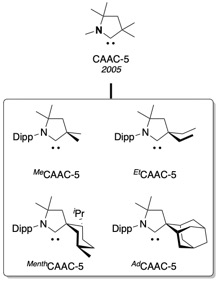
 Since 2005, the family of cyclic (alkyl)(amino)carbenes expended to encompass the functionalized FunCAACs, the BiCAACs with a bicyclic backbone, the CAAC-6s with a 6-membered backbone, and the chiral ChiCAACs used in
Since 2005, the family of cyclic (alkyl)(amino)carbenes expended to encompass the functionalized FunCAACs, the BiCAACs with a bicyclic backbone, the CAAC-6s with a 6-membered backbone, and the chiral ChiCAACs used in
 As ligand for transition metal catalysts, they distinguished themselves in ruthenium catalysis
As ligand for transition metal catalysts, they distinguished themselves in ruthenium catalysis
 In
In chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, cyclic(alkyl)(amino)carbenes (CAACs) are a family of stable singlet carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
ligands developed by Prof. Guy Bertrand anhis group
in 2005 at UC Riverside (now at UC San Diego). In marked contrast with the popular N-heterocyclic carbenes ( NHC) which possess two "amino" substituents adjacent to the "carbene" center, CAACs possess one "amino" substituent and an sp3 carbon atom "alkyl". This specific configuration makes the CAACs very good σ-donors (higher HOMO) and π-acceptors (lower LUMO) when compared to NHCs. Moreover the reduced heteroatom stabilization of the carbene center in CAACs versus NHCs also gives rise to a smaller ΔEST (48.3 vs 72.7 kcal mol-1).
Synthesis
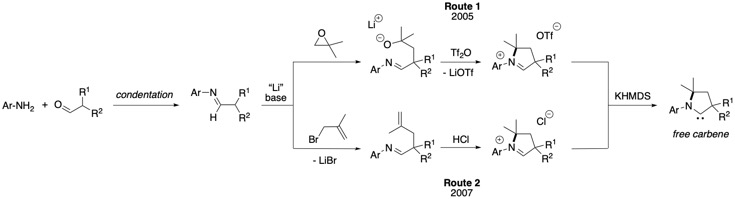
The original preparation of CAACs precursors (Route 1) begins with condensation of 2,6-diisopropylaniline and 2-methylpropanal.Deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
of this aldimine with lithium diisopropylamide
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula . It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature ...
gives an aza-allyl anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
, which ring opens 1,2-epoxy-2-methylpro-pane. The resulting lithium alkoxide is then treated with triflic anhydride
Trifluoromethanesulfonic anhydride, also known as triflic anhydride, is the chemical compound with the formula (CF3SO2)2O. It is the acid anhydride derived from triflic acid. This compound is a strong electrophile, useful for introducing the trif ...
to generate the aldiminium salt. Another methods (Route 2) involves alkylation of the aldimine with 3-bromo-2-methylpropene to generate an alkenyl aldimine, which cyclises to the corresponding iminium salts in the presence of HCl upon heating.,, This straightforward approach allows for kilogram-scale syntheses of CAAC precursors. Finally, deprotonation of the minimum salts with potassium bis(trimethylsilyl)amide
Potassium bis(trimethylsilyl)amide (commonly abbreviated as KHMDS, Potassium(K) HexaMethylDiSilazide) or potassium hexamethyldisilazane is the chemical compound with the formula ((CH3)3Si)2NK. It is a strong, non-nucleophilic base with an approxim ...
affords the free carbene as a white solid. CAAC free carbenes are air and moisture sensitive but can be stored for weeks under an inert atmosphere.

Family of CAAC ligands
 Since 2005, the family of cyclic (alkyl)(amino)carbenes expended to encompass the functionalized FunCAACs, the BiCAACs with a bicyclic backbone, the CAAC-6s with a 6-membered backbone, and the chiral ChiCAACs used in
Since 2005, the family of cyclic (alkyl)(amino)carbenes expended to encompass the functionalized FunCAACs, the BiCAACs with a bicyclic backbone, the CAAC-6s with a 6-membered backbone, and the chiral ChiCAACs used in asymmetric catalysis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
.
Applications
In recent years, cyclic (alkyl)(amino)carbenes have found numerous applications ranging from the stabilization of highly reactive species, to homogeneous catalysis and materials., Better σ-donors and π-acceptors than the well-known N-heterocyclic carbenes (NHCs), these stable singlet carbene are well known for stabilising highly reactive species, such as highly reactive low valent complexes, and main group radicals. As ligand for transition metal catalysts, they distinguished themselves in ruthenium catalysis
As ligand for transition metal catalysts, they distinguished themselves in ruthenium catalysis ethenolysis
In organic chemistry, ethenolysis is a chemical process in which internal olefins are degraded using ethylene () as the reagent. The reaction is an example of cross metathesis. The utility of the reaction is driven by the low cost of ethylene ...
processes where CAACs proved to be superior compared to NHCs reaching up to 340000 TONs. Note that this was the first time ruthenium metathesis catalysts exhibited high performance in cross‐metathesis reactions employing ethylene gas, with activities sufficient for the industrial‐scale production of linear α‐olefins (LAOs) and other terminal‐olefin products.
More recently, CAACs have been shown by Di et al. and Thompson et al. to generate very efficient OLED
An organic light-emitting diode (OLED or organic LED), also known as organic electroluminescent (organic EL) diode, is a light-emitting diode (LED) in which the emissive electroluminescent layer is a film of organic compound that emits light i ...
s materials with d10-coinage metals. Traditionally, OLED devices rely on expensive heavy transition metals such as iridium, platinum, or ruthenium which are not sustainable. Consequently, the development of d10-coinage metal alternatives is inherently more advantageous .
It was also demonstrated that their ambiphilic nature allows them to participate in the activation of enthalpically strong E-H bonds (E: N, P, Si, …), a distinctive feature traditionally reserved to transition metals. It was also shown that bulky CAACs promote the reverse transformation,{{Cite journal, last1=Tolentino, first1=Daniel R., last2=Neale, first2=Samuel E., last3=Isaac, first3=Connie J., last4=Macgregor, first4=Stuart A., last5=Whittlesey, first5=Michael K., last6=Jazzar, first6=Rodolphe, last7=Bertrand, first7=Guy, date=2019-06-26, title=Reductive Elimination at Carbon under Steric Control, journal=Journal of the American Chemical Society, volume=141, issue=25, pages=9823–9826, doi=10.1021/jacs.9b04957, pmid=31180660, issn=0002-7863 a formal reductive elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and is ...
of E-H bonds at carbon, further delineating the parallel with transition metals
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
.
References