In
quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
, a spherically symmetric potential is a system of which the
potential
Potential generally refers to a currently unrealized ability. The term is used in a wide variety of fields, from physics to the social sciences to indicate things that are in a state where they are able to change in ways ranging from the simple r ...
only depends on the
radial distance from the spherical
center and a location in space. A particle in a spherically symmetric potential will behave accordingly to said potential and can therefore be used as an approximation, for example, of the electron in a
hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral hydrogen atom contains a single positively charged proton in the nucleus, and a single negatively charged electron bound to the nucleus by the Coulomb for ...
or of the formation of
chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s.
In the general time-independent case, the dynamics of a particle in a spherically symmetric potential are governed by a
Hamiltonian
Hamiltonian may refer to:
* Hamiltonian mechanics, a function that represents the total energy of a system
* Hamiltonian (quantum mechanics), an operator corresponding to the total energy of that system
** Dyall Hamiltonian, a modified Hamiltonian ...
of the following form:
Here,
is the mass of the particle,
is the
momentum operator, and the potential
depends only on the
vector magnitude
In mathematics, a norm is a function from a real or complex vector space to the non-negative real numbers that behaves in certain ways like the distance from the origin: it commutes with scaling, obeys a form of the triangle inequality, and ze ...
of the position vector, that is, the radial distance from the origin (hence the spherical symmetry of the problem).
To describe a particle in a spherically symmetric system, it is convenient to use
spherical coordinates
In mathematics, a spherical coordinate system specifies a given point in three-dimensional space by using a distance and two angles as its three coordinates. These are
* the radial distance along the line connecting the point to a fixed point ...
; denoted by
,
and
. The
time-independent Schrödinger equation for the system is then a
separable,
partial differential equation
In mathematics, a partial differential equation (PDE) is an equation which involves a multivariable function and one or more of its partial derivatives.
The function is often thought of as an "unknown" that solves the equation, similar to ho ...
. This means solutions to the angular dimensions of the equation can be found
independently of the radial dimension. This leaves an
ordinary differential equation
In mathematics, an ordinary differential equation (ODE) is a differential equation (DE) dependent on only a single independent variable (mathematics), variable. As with any other DE, its unknown(s) consists of one (or more) Function (mathematic ...
in terms only of the radius,
, which determines the eigenstates for the particular potential,
.
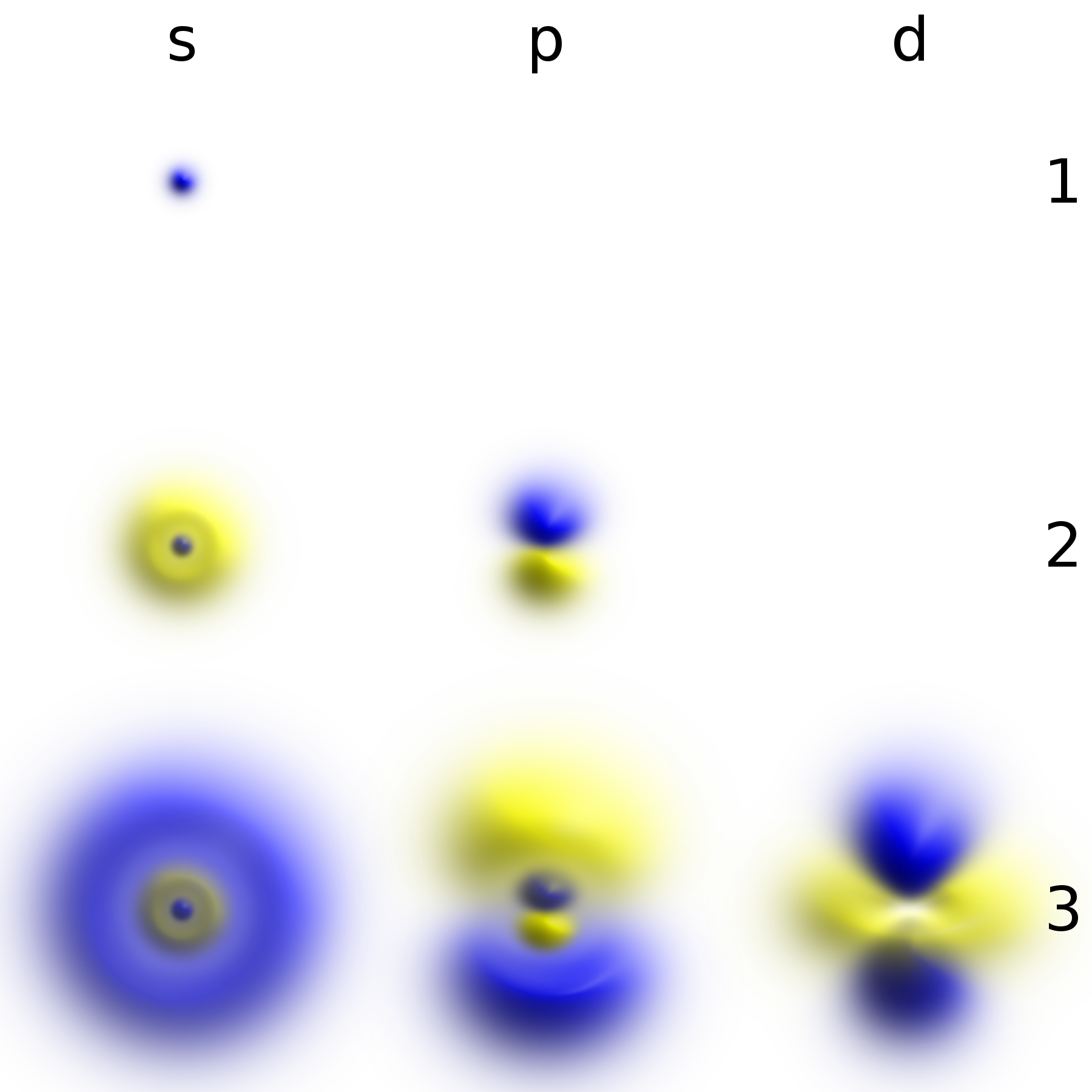
Structure of the eigenfunctions
If solved by
separation of variables
In mathematics, separation of variables (also known as the Fourier method) is any of several methods for solving ordinary differential equation, ordinary and partial differential equations, in which algebra allows one to rewrite an equation so tha ...
, the eigenstates of the
system
A system is a group of interacting or interrelated elements that act according to a set of rules to form a unified whole. A system, surrounded and influenced by its open system (systems theory), environment, is described by its boundaries, str ...
will have the form:
in which the
spherical angles
and
represent the
polar and
azimuth
An azimuth (; from ) is the horizontal angle from a cardinal direction, most commonly north, in a local or observer-centric spherical coordinate system.
Mathematically, the relative position vector from an observer ( origin) to a point ...
al angle, respectively. Those two factors of
are often grouped together as
spherical harmonics
In mathematics and physical science, spherical harmonics are special functions defined on the surface of a sphere. They are often employed in solving partial differential equations in many scientific fields. The table of spherical harmonics co ...
, so that the
eigenfunctions
In mathematics, an eigenfunction of a linear map, linear operator ''D'' defined on some function space is any non-zero function (mathematics), function f in that space that, when acted upon by ''D'', is only multiplied by some scaling factor calle ...
take the form:
The differential equation which characterises the function
is called the radial equation.
Derivation of the radial equation
The
kinetic energy
In physics, the kinetic energy of an object is the form of energy that it possesses due to its motion.
In classical mechanics, the kinetic energy of a non-rotating object of mass ''m'' traveling at a speed ''v'' is \fracmv^2.Resnick, Rober ...
operator in
spherical polar coordinates
In mathematics, a spherical coordinate system specifies a given point in three-dimensional space by using a distance and two angles as its three coordinates. These are
* the radial distance along the line connecting the point to a fixed point ...
is:
The
spherical harmonics
In mathematics and physical science, spherical harmonics are special functions defined on the surface of a sphere. They are often employed in solving partial differential equations in many scientific fields. The table of spherical harmonics co ...
satisfy
Substituting this into the
Schrödinger equation
The Schrödinger equation is a partial differential equation that governs the wave function of a non-relativistic quantum-mechanical system. Its discovery was a significant landmark in the development of quantum mechanics. It is named after E ...
we get a one-dimensional eigenvalue equation,
This equation can be reduced to an equivalent 1-D Schrödinger equation by substituting
, where
satisfies
which is precisely the one-dimensional Schrödinger equation with an effective potential given by
where
. The correction to the potential ''V''(''r'') is called the centrifugal barrier term.
If
, then near the origin,
.
Spherically symmetric Hamiltonians
Since the Hamiltonian is spherically symmetric, it is said to be invariant under rotation, ie:
Since angular momentum operators are generators of rotation, applying the Baker–Campbell–Hausdorff formula, Baker-Campbell-Hausdorff Lemma we get:
Since this equation holds for all values of
, we get that
, or that every angular momentum component commutes with the Hamiltonian.
Since
and
are such mutually commuting operators that also commute with the Hamiltonian, the wavefunctions can be expressed as
or
where
is used to label different wavefunctions.
Since
also commutes with the Hamiltonian, the energy eigenvalues in such cases are always independent of
.
Combined with the fact that
differential operators only act on the functions of
and
, it shows that if the solutions are assumed to be separable as
, the radial wavefunction
can always be chosen independent of
values. Thus the wavefunction is expressed as:
Solutions for potentials of interest
There are five cases of special importance:
#
, or solving the vacuum in the basis of spherical harmonics, which serves as the basis for other cases.
#
(finite) for
 In
In  In
In