Organocopper Compounds on:
[Wikipedia]
[Google]
[Amazon]

 Organocopper compounds is the chemistry of
Organocopper compounds is the chemistry of
 Alkenes bind to copper(I), although again generally weakly. The binding of ethylene to Cu in proteins is of broad significance in plant biology so much so that ethylene is classified as a
Alkenes bind to copper(I), although again generally weakly. The binding of ethylene to Cu in proteins is of broad significance in plant biology so much so that ethylene is classified as a


 Synthesis of Z-Fluoro alkene dipeptide isosteres,. Other effort to make this a more selective reactions includes the use of oxidation reduction condition for the reaction. Fluoride acts as a leaving group and it enhances
Synthesis of Z-Fluoro alkene dipeptide isosteres,. Other effort to make this a more selective reactions includes the use of oxidation reduction condition for the reaction. Fluoride acts as a leaving group and it enhances 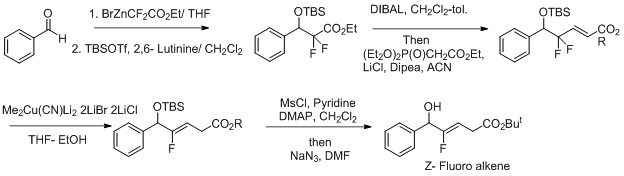
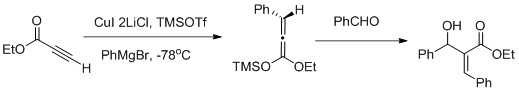 Muller and collaborators reported a vicinal functionalization of α,β- acetylenic esters using a carbocupration/Mukaiyama aldol reaction sequence (as shown in fig. above) carbocupration favors the formation of the Z- aldol.
Muller and collaborators reported a vicinal functionalization of α,β- acetylenic esters using a carbocupration/Mukaiyama aldol reaction sequence (as shown in fig. above) carbocupration favors the formation of the Z- aldol.

 Organocopper compounds is the chemistry of
Organocopper compounds is the chemistry of organometallic compounds
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
containing a carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
to copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
. Organocopper chemistry is the study of organocopper compounds describing their physical properties, synthesis and reactions. They are reagents in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
.
The first organocopper compound, the explosive copper(I) acetylide
Copper(I) acetylide, or cuprous acetylide, is a chemical compound with the formula Cu2 C2. Although never characterized by X-ray crystallography, the material has been claimed at least since 1856. One form is claimed to be a monohydrate with for ...
Cu2C2 (Cu−C≡C−Cu), was synthesized by Rudolf Christian Böttger Rudolf Christian Böttger (28 April 1806 – 29 April 1881) was a German inorganic chemist. He conducted most of his research at the University of Frankfurt am Main. He is credited with discovery of nitrocellulose in 1846, independently to Schönbe ...
in 1859 by passing acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
gas through a solution of copper(I) chloride
Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear gre ...
:
:C2H2 + 2 CuCl → Cu2C2 + 2 HCl
Structure and bonding
Organocopper compounds are diverse in structure and reactivity, but organocopper compounds are largely limited inoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
s to copper(I), sometimes denoted Cu+. As a d10 metal center, it is related to Ni(0), but owing to its higher oxidation state, it engages in less pi-backbonding. Organic derivatives of Cu(II) and Cu(III) are invoked as intermediates but rarely isolated or even observed. In terms of geometry, copper(I) adopts symmetrical structures, in keeping with its spherical electronic shell. Typically one of three coordination geometries is adopted: linear 2-coordinate, trigonal 3-coordinate, and tetrahedral 4-coordinate. Organocopper compounds form complexes with a variety of soft ligands such as alkylphosphines (R3P), thioethers (R2S), and cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
(CN−).
Simple complexes with CO, alkene, and Cp ligands
Copper(I) salts have long been known to bind CO, albeit weakly. A representative complex is CuCl(CO), which is polymeric. In contrast to classical metal carbonyls, pi-backbonding is not strong in these compounds. Alkenes bind to copper(I), although again generally weakly. The binding of ethylene to Cu in proteins is of broad significance in plant biology so much so that ethylene is classified as a
Alkenes bind to copper(I), although again generally weakly. The binding of ethylene to Cu in proteins is of broad significance in plant biology so much so that ethylene is classified as a plant hormone
Plant hormone (or phytohormones) are signal molecules, produced within plants, that occur in extremely low concentrations. Plant hormones control all aspects of plant growth and development, from embryogenesis, the regulation of organ size, pa ...
. Its presence, detected by the Cu-protein, affects ripening and many other developments.
Although copper does not form a metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions (, abbreviated Cp) bound to a metallic element, metal center (M) in the oxidation state II, with the resulting general formula Closely related to the metallocenes are ...
, half-sandwich complexes can be produced. One such derivative is π-cyclopentadienyl(triethylphosphine)copper(I).
Alkyl and aryl copper compounds
Alkyl and aryl copper(I) compounds
Copper halides react withorganolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s to give organocopper compounds. The area was pioneered by Henry Gilman
Henry Gilman (May 9, 1893 – November 7, 1986) was an American organic chemist known as the father of organometallic chemistry, the field within which his most notable work was done. He discovered the Gilman reagent, which bears his name.
Earl ...
, who reported methylcopper in 1936. Thus, phenylcopper
Phenylcopper is an organometallic chemical compound of copper. Its chemical formula is .
Synthesis
Phenylcopper was the first known organocopper compound and was first prepared in 1923 from phenylmagnesium iodide and copper(I) iodide and in 1936 ...
is prepared by reaction of phenyllithium
Phenyllithium or lithobenzene is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic synthes ...
with copper(I) bromide
Copper(I) bromide is the chemical compound with the formula CuBr. This diamagnetic solid adopts a polymeric structure akin to that for zinc sulfide. The compound is widely used in the synthesis of organic compounds and as a lasing medium in c ...
in diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liq ...
. Grignard reagents can be used in place of organolithium compounds. Gilman also investigated the dialkylcuprates. These are obtained by combining two equivalent of RLi with Cu(I) salts. Alternatively, these cuprates are prepared from oligomeric neutral organocopper compounds by treatment with one equivalent of organolithium reagent.
Compounds of the type uR''n''sup>(''n''-1)- are reactive towards oxygen and water, forming copper(I) oxide
Copper(I) oxide or cuprous oxide is the inorganic compound with the formula Cu2O. It is one of the principal oxides of copper, the other being or copper(II) oxide or cupric oxide (CuO). This red-coloured solid is a component of some antifoulin ...
. They also tend to be thermally unstable, which can be useful in certain coupling reactions. Despite or because of these difficulties, organocopper reagents are frequently generated and consumed in situ
''In situ'' (; often not italicized in English) is a Latin phrase that translates literally to "on site" or "in position." It can mean "locally", "on site", "on the premises", or "in place" to describe where an event takes place and is used in ...
with no attempt to isolate them. They are used in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
as alkylating reagent
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
s because they exhibit greater functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
tolerance than corresponding Grignard and organolithium reagents. The electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
of copper is much higher than its next-door neighbor in the group 12 element
Group 12, by modern IUPAC numbering, is a group of chemical elements in the periodic table. It includes zinc (Zn), cadmium (Cd), mercury (Hg), and copernicium (Cn). Formerly this group was named ''IIB'' (pronounced as "group two B", as the "II" ...
s, zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
, suggesting diminished nucleophilicity
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
for its carbon ligands.
Copper salts react with terminal alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s to form the acetylide
In organometallic chemistry, acetylide refers to chemical compounds with the chemical formulas and , where M is a metal. The term is used loosely and can refer to substituted acetylides having the general structure (where R is an organic side c ...
s.
Alkyl halides react with organocopper compounds with inversion of configuration. On the other hand, reactions of organocopper compound with alkenyl halides proceed with retention of subtrate’s configuration.
Organocopper compounds couple with aryl halides:
:
Structures
Alkyl and aryl copper complexes aggregate both in crystalline form and in solution. Aggregation is especially evident for charge-neutral organocopper compounds, i.e. species with theempirical formula
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is th ...
(RCu), which adopt cyclic structures. Since each copper center requires at least two ligands, the organic group is a bridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually r ...
. This effect is illustrated by the structure of mesitylcopper, which is a pentamer. A cyclic structure is also seen for CuCH2SiMe3, first 1:1 organocopper compound to be analyzed by X-ray crystallography
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The wor ...
(1972 by Lappert). This compound is relatively stable because the bulky trimethylsilyl
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom minus;Si(CH3)3 which is in turn bonded to the rest of a molecule. This structural group is ch ...
groups provide steric protection. It is a tetramer
A tetramer () (''tetra-'', "four" + '' -mer'', "parts") is an oligomer formed from four monomers or subunits. The associated property is called ''tetramery''. An example from inorganic chemistry is titanium methoxide with the empirical formula Ti ...
, forming an 8-membered ring with alternating Cu-C bonds. In addition the four copper atoms form a planar Cu4 ring based on three-center two-electron bond
A three-center two-electron (3c–2e) bond is an electron-deficient chemical bond where three atoms share two electrons. The combination of three atomic orbitals form three molecular orbitals: one bonding, one ''non''-bonding, and one ''anti''- ...
s. The copper to copper bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of ...
is 242 pm compared to 256 pm in bulk copper. In ''pentamesitylpentacopper'' a 5-membered copper ring is formed, similar to (2,4,6-trimethylphenyl)gold, and ''pentafluorophenylcopper'' is a tetramer.
:
Lithium dimethylcuprate is a dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ...
in diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liq ...
, forming an 8-membered ring with two lithium atoms linking two methyl groups. Similarly, lithium diphenylcuprate forms a dimeric etherate, CuPh2)sub>2, in the solid state.
Alkyl and aryl copper(III) compounds
The involvement of the otherwise rare Cu(III) oxidation state has been demonstrated in theconjugate addition
Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarit ...
of the Gilman reagent
A Gilman reagent is a lithium and copper ( diorganocopper) reagent compound, R2CuLi, where R is an alkyl or aryl. These reagents are useful because, unlike related Grignard reagents and organolithium reagents, they react with organic halides to ...
to an enone: In a so-called rapid-injection NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with ...
experiment at -100 °C, the Gilman reagent Me2CuLi (stabilized by lithium iodide
Lithium iodide, or LiI, is a compound of lithium and iodine. When exposed to air, it becomes yellow in color, due to the oxidation of iodide to iodine. It crystallizes in the NaCl motif. It can participate in various hydrates.Wietelmann, Ulrich an ...
) was introduced to cyclohexenone (1) enabling the detection of the copper — alkene pi complex
The number (; spelled out as "pi") is a mathematical constant that is the ratio of a circle's circumference to its diameter, approximately equal to 3.14159. The number appears in many formulas across mathematics and physics. It is an irratio ...
2. On subsequent addition of trimethylsilyl cyanide
Trimethylsilyl cyanide is the chemical compound with the formula (CH3)3SiCN. This volatile liquid consists of a cyanide group, that is CN, attached to a trimethylsilyl group. The molecule is used in organic synthesis as the equivalent of hydrogen ...
the Cu(III) species 3 is formed (indefinitely stable at that temperature) and on increasing the temperature to -80 °C the conjugate addition product 4. According to an accompanying in silico
In biology and other experimental sciences, an ''in silico'' experiment is one performed on computer or via computer simulation. The phrase is pseudo-Latin for 'in silicon' (correct la, in silicio), referring to silicon in computer chips. It ...
experiments the Cu(III) intermediate has a square planar molecular geometry
The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the corne ...
with the cyano
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ...
group in cis orientation with respect to the cyclohexenyl methine
In organic chemistry, a methine group or methine bridge is a trivalent functional group , derived formally from methane. It consists of a carbon atom bound by two single covalent bond, bonds and one double bond, where one of the single bonds is t ...
group and anti-parallel to the methine proton. With other ligands than the cyano group
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ...
this study predicts room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
stable Cu(III) compounds.
:
Reactions of organocuprates
Cross-coupling reactions
Prior to the development ofpalladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
-catalyzed cross coupling reaction
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = ...
s, copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
was the preferred catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
for almost a century. Palladium offers a faster, more selective reaction. However, in recent years copper has reemerged as a synthetically useful metal, because of its lower cost and because it is an eco-friendly metal.
Reactions of R2CuLi with alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely ...
s R'-X give the coupling product:
:R2CuLi + R'X → R-R' + CuR + LiX
The reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
involves oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...
(OA) of the alkyl halide to Cu(I), forming a planar
Planar is an adjective meaning "relating to a plane (geometry)".
Planar may also refer to:
Science and technology
* Planar (computer graphics), computer graphics pixel information from several bitplanes
* Planar (transmission line technologies), ...
Cu(III) intermediate, followed by reductive elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and is ...
(RE). The nucleophilic attack is the rate-determining step. In the substitution of iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine defic ...
, a single-electron transfer mechanism is proposed (see figure).
:
Many electrophiles participate in this reaction. The approximate order of reactivity, beginning with the most reactive, is as follows: acid chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
s > aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s > tosylate
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula –. It consists of a tolyl group, –, joined to a sulfonyl group, ––, with the open valence on s ...
s ~ epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for ...
s > iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine defic ...
s > bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardant ...
s > chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts ...
s > ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s > ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
s > nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix ''cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including met ...
s >> alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s
Generally the OA-RE mechanism is analogous to that of palladium-catalyzed cross coupling reactions. One difference between copper and palladium is that copper can undergo single-electron transfer processes.Posner, G. H. 2011. Substitution Reactions Using Organocopper Reagents. Organic Reactions. 22:2:253–400
Coupling reactions
Oxidative coupling is the coupling of copper acetylides to conjugated alkynes in theGlaser coupling
The Glaser coupling is a type of coupling reaction. It is by far the oldest acetylenic coupling and is based on cuprous salts like copper(I) chloride or copper(I) bromide and an additional oxidant like oxygen. The base in its original scope is amm ...
(for example in the synthesis of cyclooctadecanonaene
Cyclooctadecanonaene or 8nnulene is an organic compound with chemical formula . It belongs to the class of highly conjugated compounds known as annulenes and is aromatic. The usual isomer that 8nnulene refers to is the most stable one, containi ...
) or to aryl halides in the Castro-Stephens Coupling.
Reductive coupling is a coupling reaction A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = ...
of aryl halides with a stoichiometric equivalent of copper metal that occurs in the Ullmann reaction
The Ullmann reaction or Ullmann coupling is a coupling reaction between aryl halides. Traditionally this reaction is effected by copper, but palladium and nickel are also effective catalysts. The reaction is named after Fritz Ullmann.
Mechanism
...
. In an example of a present-day cross coupling reaction called decarboxylative coupling, a catalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
amount of Cu(I) displaces a carboxyl
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
group forming the arylcopper (ArCu) intermediate. Simultaneously, a palladium catalyst converts an aryl bromide to the organopalladium intermediate (Ar'PdBr), and on transmetallation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
the biaryl is formed from ArPdAr'.
:
Redox neutral coupling is the coupling of terminal alkynes with halo-alkynes with a copper(I) salt in the Cadiot-Chodkiewicz coupling. Thermal coupling of two organocopper compounds is also possible.
Carbocupration
Carbocupration is anucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions di ...
of organocopper reagents (R-Cu) to acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
or terminal alkynes
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
resulting in an alkenylcopper compound (RC=C−Cu). It is a special case of carbometalation
A carbometalation is any reaction where a carbon-metal bond reacts with a carbon-carbon π-bond to produce a new carbon-carbon σ-bond and a carbon-metal σ-bond. The resulting carbon-metal bond can undergo further carbometallation reactions (oli ...
and also called the Normant reaction.

Synthetic applications
*The Chan-Lam coupling enables the formation of aryl carbon-hetoroatom bonds. It involves coupling ofboronic acids
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, membe ...
, stannanes, or siloxanes with NH- or OH-containing substrates.
*Ullmann reaction
The Ullmann reaction or Ullmann coupling is a coupling reaction between aryl halides. Traditionally this reaction is effected by copper, but palladium and nickel are also effective catalysts. The reaction is named after Fritz Ullmann.
Mechanism
...
involves copper-mediated reactions of aryl halides. Two types of Ullmann reaction are recognized:
*#Classic copper-promoted synthesis of symmetric biaryl compounds)
*#Copper-promoted nucleophilic aromatic substitution.
*Sonogashira coupling
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vi ...
reaction, which utilizes both copper and palladium, entails the coupling of aryl and/or vinyl halides with terminal alkynes.
Reducing agents
Copper hydrides are specialized reagents used occasionally asreducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth meta ...
. The best known copper hydride is called Stryker's reagent
Stryker's reagent ( PPh3)CuHsub>6), also known as the Osborn complex, is a hexameric copper hydride ligated with triphenylphosphine. It is a brick red, air-sensitive solid. Stryker's reagent is a mildly hydridic reagent, used in homogeneous cat ...
, a cluster compound with the formula PPh3)CuHsub>6. It reduces the alkene α,β-unsaturated carbonyl compounds
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
.
The Buchwald reaction is a copper-catalyzed asymmetric reduction of activated alkenes. The reagent is generated in situ from copper(I) NHC complex. The hydride equivalents are provided by a silane
Silane is an inorganic compound with chemical formula, . It is a colourless, pyrophoric, toxic gas with a sharp, repulsive smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Sila ...
.
: Synthesis of Z-Fluoro alkene dipeptide isosteres,. Other effort to make this a more selective reactions includes the use of oxidation reduction condition for the reaction. Fluoride acts as a leaving group and it enhances
Synthesis of Z-Fluoro alkene dipeptide isosteres,. Other effort to make this a more selective reactions includes the use of oxidation reduction condition for the reaction. Fluoride acts as a leaving group and it enhances regioselectivity
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base ...
in the transformation the Z- fluoroalkene.

Cu alkylation reaction
Generally, the alkylation reaction of organocopper reagents proceed via gamma- alkylation. Cis- gamma attack occurs better in cyclohexyl carbamate due to sterics. The reaction is reported to be favorable in ethereal solvents. This method was proved to be very effective for the oxidative coupling of amines and alkyl, including tertbutyl, and aryl halides.Vicinal functionalization reactions
Vicinal functionalization using a Carbocupration- Mukaiyama aldol reaction sequence: Muller, A.J.; Jennings, M.P. Vicinal Functionalization of propionilate Esters via Tandem Catalytic Carbocupration-Mukaiyama Aldol Reaction sequence. Org. Lett. 2008, 10, 1649-1652 Muller and collaborators reported a vicinal functionalization of α,β- acetylenic esters using a carbocupration/Mukaiyama aldol reaction sequence (as shown in fig. above) carbocupration favors the formation of the Z- aldol.
Muller and collaborators reported a vicinal functionalization of α,β- acetylenic esters using a carbocupration/Mukaiyama aldol reaction sequence (as shown in fig. above) carbocupration favors the formation of the Z- aldol.
Further reading
* *References
{{ChemicalBondsToCarbon