Molecular Crystal on:
[Wikipedia]
[Google]
[Amazon]
 A molecular solid is a solid consisting of discrete molecules. The cohesive forces that bind the molecules together are van der Waals forces,
A molecular solid is a solid consisting of discrete molecules. The cohesive forces that bind the molecules together are van der Waals forces,
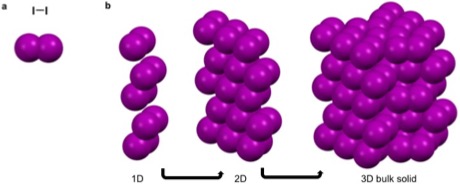 Argon, is a noble gas that has a full octet, no charge, and is nonpolar. These characteristics make it unfavorable for argon to partake in metallic, covalent, and ionic bonds as well as most intermolecular interactions. It can though partake in van der Waals and London dispersion forces. These weak self-interactions are
Argon, is a noble gas that has a full octet, no charge, and is nonpolar. These characteristics make it unfavorable for argon to partake in metallic, covalent, and ionic bonds as well as most intermolecular interactions. It can though partake in van der Waals and London dispersion forces. These weak self-interactions are
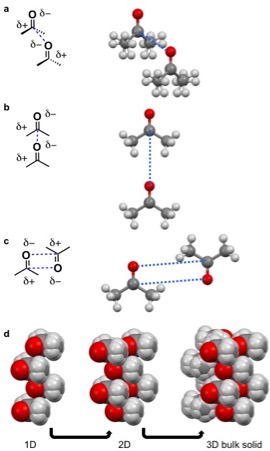 For acetone dipole-dipole interactions are a major driving force behind the structure of its crystal lattice. The negative dipole is caused by oxygen. Oxygen is more electronegative than carbon and hydrogen, causing a partial negative (δ-) and positive charge (δ+) on the oxygen and remainder of the molecule, respectively. The δ- orienttowards the δ+ causing the acetone molecules to prefer to align in a few configurations in a δ- to δ+ orientation (pictured left). The dipole-dipole and other intermolecular interactions align to minimize energy in the solid state and determine the crystal lattice structure.
For acetone dipole-dipole interactions are a major driving force behind the structure of its crystal lattice. The negative dipole is caused by oxygen. Oxygen is more electronegative than carbon and hydrogen, causing a partial negative (δ-) and positive charge (δ+) on the oxygen and remainder of the molecule, respectively. The δ- orienttowards the δ+ causing the acetone molecules to prefer to align in a few configurations in a δ- to δ+ orientation (pictured left). The dipole-dipole and other intermolecular interactions align to minimize energy in the solid state and determine the crystal lattice structure.
 A quadrupole, like a dipole, is a permanent pole but the electric field of the molecule is not linear as in acetone, but in two dimensions. Examples of molecular solids with quadrupoles are octafluoronaphthalene and naphthalene. Naphthalene consists of two joined conjugated rings. The electronegativity of the atoms of this ring system and conjugation cause a ring current resulting in a quadrupole. For naphthalene, this quadrupole manifests in a δ- and δ+ accumulating within and outside the ring system, respectively. Naphthalene assembles through the coordination of δ- of one molecules to the δ+ of another molecule. This results in 1D columns of naphthalene in a herringbone configuration. These columns then stack into 2D layers and then 3D bulk materials. Octafluoronaphthalene follows this path of organization to build bulk material except the δ- and δ+ are on the exterior and interior of the ring system, respectively.
A quadrupole, like a dipole, is a permanent pole but the electric field of the molecule is not linear as in acetone, but in two dimensions. Examples of molecular solids with quadrupoles are octafluoronaphthalene and naphthalene. Naphthalene consists of two joined conjugated rings. The electronegativity of the atoms of this ring system and conjugation cause a ring current resulting in a quadrupole. For naphthalene, this quadrupole manifests in a δ- and δ+ accumulating within and outside the ring system, respectively. Naphthalene assembles through the coordination of δ- of one molecules to the δ+ of another molecule. This results in 1D columns of naphthalene in a herringbone configuration. These columns then stack into 2D layers and then 3D bulk materials. Octafluoronaphthalene follows this path of organization to build bulk material except the δ- and δ+ are on the exterior and interior of the ring system, respectively.
 A hydrogen bond is a specific dipole where a hydrogen atom has a partial positive charge (δ+) to due a neighboring electronegative atom or functional group. Hydrogen bonds are amongst the strong intermolecular interactions know other than ion-dipole interactions. For intermolecular hydrogen bonds the δ+ hydrogen interacts with a δ- on an adjacent molecule. Examples of molecular solids that hydrogen bond are water, amino acids, and acetic acid. For acetic acid, the hydrogen (δ+) on the
A hydrogen bond is a specific dipole where a hydrogen atom has a partial positive charge (δ+) to due a neighboring electronegative atom or functional group. Hydrogen bonds are amongst the strong intermolecular interactions know other than ion-dipole interactions. For intermolecular hydrogen bonds the δ+ hydrogen interacts with a δ- on an adjacent molecule. Examples of molecular solids that hydrogen bond are water, amino acids, and acetic acid. For acetic acid, the hydrogen (δ+) on the  A halogen bond is when an electronegative
A halogen bond is when an electronegative
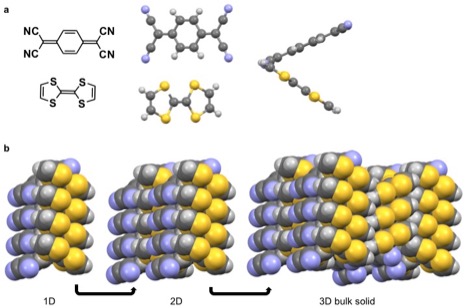 Coulombic interactions are manifested in some molecular solids. A well-studied example is the radical ion salt TTF-TCNQ with a conductivity of 5 x 102 Ω−1 cm−1, much closer to copper (ρ = 6 x 105 Ω−1 cm−1) than many molecular solids. The coulombic interaction in TTF-TCNQ stems from the large partial negative charge (δ = -0.59) on the cyano- moiety on TCNQ at room temperature. For reference, a completely charged molecule δ = ±1. This partial negative charge leads to a strong interaction with the thio- moiety of the TTF. The strong interaction leads to favorable alignment of these functional groups adjacent to each other in the solid state. While π-π interactions cause the TTF and TCNQ to stack in separate columns.
Coulombic interactions are manifested in some molecular solids. A well-studied example is the radical ion salt TTF-TCNQ with a conductivity of 5 x 102 Ω−1 cm−1, much closer to copper (ρ = 6 x 105 Ω−1 cm−1) than many molecular solids. The coulombic interaction in TTF-TCNQ stems from the large partial negative charge (δ = -0.59) on the cyano- moiety on TCNQ at room temperature. For reference, a completely charged molecule δ = ±1. This partial negative charge leads to a strong interaction with the thio- moiety of the TTF. The strong interaction leads to favorable alignment of these functional groups adjacent to each other in the solid state. While π-π interactions cause the TTF and TCNQ to stack in separate columns.
 A molecular solid is a solid consisting of discrete molecules. The cohesive forces that bind the molecules together are van der Waals forces,
A molecular solid is a solid consisting of discrete molecules. The cohesive forces that bind the molecules together are van der Waals forces, dipole-dipole interactions
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
, quadrupole interactions, π-π interactions, hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ing, halogen bonding, London dispersion forces, and in some molecular solids, coulombic interactions. Van der Waals, dipole interactions, quadrupole interactions, π-π interactions, hydrogen bonding, and halogen bonding (2-127 kJ mol−1) are typically much weaker than the forces holding together other solids: metallic ( metallic bonding, 400-500 kJ mol−1), ionic ( Coulomb’s forces, 700-900 kJ mol−1), and network solids A network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) is a chemical compound (or element) in which the atoms are bonded by covalent bonds in a continuous network extending throughout the mat ...
(covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
s, 150-900 kJ mol−1). Intermolecular interactions, typically do not involve delocalized electrons, unlike metallic and certain covalent bonds. Exceptions are charge-transfer complex
In chemistry, a charge-transfer (CT) complex or electron-donor-acceptor complex describes a type of supramolecular assembly of two or more molecules or ions. The assembly consists of two molecules that self-attract through electrostatic forces ...
es such as the tetrathiafulvane-tetracyanoquinodimethane (TTF-TCNQ), a radical ion salt. These differences in the strength of force (i.e. covalent vs. van der Waals) and electronic characteristics (i.e. delocalized electrons) from other types of solids give rise to the unique mechanical
Mechanical may refer to:
Machine
* Machine (mechanical), a system of mechanisms that shape the actuator input to achieve a specific application of output forces and movement
* Mechanical calculator, a device used to perform the basic operations of ...
, electronic, and thermal properties of molecular solids.
Molecular solids are poor electrical conductors, although some, such as TTF-TCNQ
Tetrathiafulvalene is an organosulfur compound with the formula (. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, , by replacement of four CH groups wit ...
are semiconductors (ρ = 5 x 102 Ω−1 cm−1). They are still substantially less than the conductivity of copper (ρ = 6 x 105 Ω−1 cm−1). Molecular solids tend to have lower fracture toughness
In materials science, fracture toughness is the critical stress intensity factor of a sharp crack where propagation of the crack suddenly becomes rapid and unlimited. A component's thickness affects the constraint conditions at the tip of a c ...
(sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula .
For human consumption, sucrose is extracted and refined ...
, KIc = 0.08 MPa m1/2) than metal ( iron, KIc = 50 MPa m1/2), ionic (sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
, KIc = 0.5 MPa m1/2), and covalent solids ( diamond, KIc = 5 MPa m1/2). Molecular solids have low melting (Tm) and boiling (Tb) points compared to metal (iron), ionic (sodium chloride), and covalent solids (diamond). Examples of molecular solids with low melting and boiling temperatures include argon, water, naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromati ...
, nicotine
Nicotine is a naturally produced alkaloid in the nightshade family of plants (most predominantly in tobacco and ''Duboisia hopwoodii'') and is widely used recreationally as a stimulant and anxiolytic. As a pharmaceutical drug, it is used fo ...
, and caffeine (see table below). The constituents of molecular solids range in size from condensed monatomic gases to small molecules (i.e. naphthalene and water) to large molecules with tens of atoms (i.e. fullerene with 60 carbon atoms).
Composition and structure
Molecular solids may consist of single atoms,diatomic
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. Ot ...
, and/or polyatomic molecules. The intermolecular interactions between the constituents dictate how the crystal lattice
In geometry and crystallography, a Bravais lattice, named after , is an infinite array of discrete points generated by a set of discrete translation operations described in three dimensional space by
: \mathbf = n_1 \mathbf_1 + n_2 \mathbf_2 + n ...
of the material is structured. All atoms and molecules can partake in van der Waals and London dispersion forces (sterics
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
). It is the lack or presence of other intermolecular interactions based on the atom or molecule that affords materials unique properties.
Van der Waals forces
 Argon, is a noble gas that has a full octet, no charge, and is nonpolar. These characteristics make it unfavorable for argon to partake in metallic, covalent, and ionic bonds as well as most intermolecular interactions. It can though partake in van der Waals and London dispersion forces. These weak self-interactions are
Argon, is a noble gas that has a full octet, no charge, and is nonpolar. These characteristics make it unfavorable for argon to partake in metallic, covalent, and ionic bonds as well as most intermolecular interactions. It can though partake in van der Waals and London dispersion forces. These weak self-interactions are isotropic
Isotropy is uniformity in all orientations; it is derived . Precise definitions depend on the subject area. Exceptions, or inequalities, are frequently indicated by the prefix ' or ', hence ''anisotropy''. ''Anisotropy'' is also used to describe ...
and result in the long-range ordering of the atoms into face centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal_structure#Unit_cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There ...
packing when cooled below -189.3. Similarly iodine, a linear diatomic molecule has a net dipole of zero and can only partake in van der Waals interactions that are fairly isotropic. This results in the bipyramidal symmetry.
Dipole-dipole and quadrupole interactions
 For acetone dipole-dipole interactions are a major driving force behind the structure of its crystal lattice. The negative dipole is caused by oxygen. Oxygen is more electronegative than carbon and hydrogen, causing a partial negative (δ-) and positive charge (δ+) on the oxygen and remainder of the molecule, respectively. The δ- orienttowards the δ+ causing the acetone molecules to prefer to align in a few configurations in a δ- to δ+ orientation (pictured left). The dipole-dipole and other intermolecular interactions align to minimize energy in the solid state and determine the crystal lattice structure.
For acetone dipole-dipole interactions are a major driving force behind the structure of its crystal lattice. The negative dipole is caused by oxygen. Oxygen is more electronegative than carbon and hydrogen, causing a partial negative (δ-) and positive charge (δ+) on the oxygen and remainder of the molecule, respectively. The δ- orienttowards the δ+ causing the acetone molecules to prefer to align in a few configurations in a δ- to δ+ orientation (pictured left). The dipole-dipole and other intermolecular interactions align to minimize energy in the solid state and determine the crystal lattice structure.
 A quadrupole, like a dipole, is a permanent pole but the electric field of the molecule is not linear as in acetone, but in two dimensions. Examples of molecular solids with quadrupoles are octafluoronaphthalene and naphthalene. Naphthalene consists of two joined conjugated rings. The electronegativity of the atoms of this ring system and conjugation cause a ring current resulting in a quadrupole. For naphthalene, this quadrupole manifests in a δ- and δ+ accumulating within and outside the ring system, respectively. Naphthalene assembles through the coordination of δ- of one molecules to the δ+ of another molecule. This results in 1D columns of naphthalene in a herringbone configuration. These columns then stack into 2D layers and then 3D bulk materials. Octafluoronaphthalene follows this path of organization to build bulk material except the δ- and δ+ are on the exterior and interior of the ring system, respectively.
A quadrupole, like a dipole, is a permanent pole but the electric field of the molecule is not linear as in acetone, but in two dimensions. Examples of molecular solids with quadrupoles are octafluoronaphthalene and naphthalene. Naphthalene consists of two joined conjugated rings. The electronegativity of the atoms of this ring system and conjugation cause a ring current resulting in a quadrupole. For naphthalene, this quadrupole manifests in a δ- and δ+ accumulating within and outside the ring system, respectively. Naphthalene assembles through the coordination of δ- of one molecules to the δ+ of another molecule. This results in 1D columns of naphthalene in a herringbone configuration. These columns then stack into 2D layers and then 3D bulk materials. Octafluoronaphthalene follows this path of organization to build bulk material except the δ- and δ+ are on the exterior and interior of the ring system, respectively.
Hydrogen and halogen bonding
 A hydrogen bond is a specific dipole where a hydrogen atom has a partial positive charge (δ+) to due a neighboring electronegative atom or functional group. Hydrogen bonds are amongst the strong intermolecular interactions know other than ion-dipole interactions. For intermolecular hydrogen bonds the δ+ hydrogen interacts with a δ- on an adjacent molecule. Examples of molecular solids that hydrogen bond are water, amino acids, and acetic acid. For acetic acid, the hydrogen (δ+) on the
A hydrogen bond is a specific dipole where a hydrogen atom has a partial positive charge (δ+) to due a neighboring electronegative atom or functional group. Hydrogen bonds are amongst the strong intermolecular interactions know other than ion-dipole interactions. For intermolecular hydrogen bonds the δ+ hydrogen interacts with a δ- on an adjacent molecule. Examples of molecular solids that hydrogen bond are water, amino acids, and acetic acid. For acetic acid, the hydrogen (δ+) on the alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
moiety
Moiety may refer to:
Chemistry
* Moiety (chemistry), a part or functional group of a molecule
** Moiety conservation, conservation of a subgroup in a chemical species
Anthropology
* Moiety (kinship), either of two groups into which a society is ...
of the carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
hydrogen bonds with other the carbonyl moiety (δ-) of the carboxylic on the adjacent molecule. This hydrogen bond leads a string of acetic acid molecules hydrogen bonding to minimize free energy. These strings of acetic acid molecules then stack together to build solids.
 A halogen bond is when an electronegative
A halogen bond is when an electronegative halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
participates in a noncovalent interaction with a less electronegative atom on an adjacent molecule. Examples of molecular solids that halogen bond are hexachlorobenzene
Hexachlorobenzene, or perchlorobenzene, is an organochloride with the molecular formula C6Cl6. It is a fungicide formerly used as a seed treatment, especially on wheat to control the fungal disease bunt. It has been banned globally under the Sto ...
and a cocrystal of bromine 1,4-dioxane
1,4-Dioxane () is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers ( 1,2- ...
. For the second example, the δ- bromine atom in the diatomic bromine molecule is aligning with the less electronegative oxygen in the 1,4-dioxane. The oxygen in this case is viewed as δ+ compared to the bromine atom. This coordination results in a chain-like organization that stack into 2D and then 3D.
Coulombic interactions
 Coulombic interactions are manifested in some molecular solids. A well-studied example is the radical ion salt TTF-TCNQ with a conductivity of 5 x 102 Ω−1 cm−1, much closer to copper (ρ = 6 x 105 Ω−1 cm−1) than many molecular solids. The coulombic interaction in TTF-TCNQ stems from the large partial negative charge (δ = -0.59) on the cyano- moiety on TCNQ at room temperature. For reference, a completely charged molecule δ = ±1. This partial negative charge leads to a strong interaction with the thio- moiety of the TTF. The strong interaction leads to favorable alignment of these functional groups adjacent to each other in the solid state. While π-π interactions cause the TTF and TCNQ to stack in separate columns.
Coulombic interactions are manifested in some molecular solids. A well-studied example is the radical ion salt TTF-TCNQ with a conductivity of 5 x 102 Ω−1 cm−1, much closer to copper (ρ = 6 x 105 Ω−1 cm−1) than many molecular solids. The coulombic interaction in TTF-TCNQ stems from the large partial negative charge (δ = -0.59) on the cyano- moiety on TCNQ at room temperature. For reference, a completely charged molecule δ = ±1. This partial negative charge leads to a strong interaction with the thio- moiety of the TTF. The strong interaction leads to favorable alignment of these functional groups adjacent to each other in the solid state. While π-π interactions cause the TTF and TCNQ to stack in separate columns.
Allotropes
One form of an element may be a molecular solid, but another form of that same element may not be a molecular solid. For example, solid phosphorus can crystallize as differentallotrope
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the ...
s called "white", "red", and "black" phosphorus. White phosphorus forms molecular crystals composed of tetrahedral P4 molecules. Heating at ambient pressure to 250 °C or exposing to sunlight
Sunlight is a portion of the electromagnetic radiation given off by the Sun, in particular infrared, visible, and ultraviolet light. On Earth, sunlight is scattered and filtered through Earth's atmosphere, and is obvious as daylight when t ...
converts white phosphorus to red phosphorus where the P4 tetrahedra are no longer isolated, but connected by covalent bonds into polymer-like chains. Heating white phosphorus under high (GPa) pressures converts it to black phosphorus which has a layered, graphite-like structure.
The structural transitions in phosphorus are reversible: upon releasing high pressure, black phosphorus gradually converts into the red phosphorus, and by vaporizing red phosphorus at 490 °C in an inert atmosphere and condensing the vapor, covalent red phosphorus can be transformed into the molecular solid, white phosphorus.
Similarly, yellow arsenic is a molecular solid composed of As4 units. Some forms of sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
and selenium are composed of S8 (or Se8) units and are molecular solids at ambient conditions, but converted into covalent allotropes having atomic chains extending throughout the crystal.
Properties
Since molecular solids are held together by relatively weak forces they tend to have low melting and boiling points, low mechanical strength, low electrical conductivity, and poor thermal conductivity.it will Also, depending on the structure of the molecule the intermolecular forces may have directionality leading to anisotropy of certain properties.Melting and boiling points
The characteristic melting point of metals and ionic solids is ~ 1000 °C and greater, while molecular solids typically melt closer to 300 °C (see table), thus many corresponding substances are either liquid (ice) or gaseous (oxygen) at room temperature. This is due to the elements involved, the molecules they form, and the weak intermolecular interactions of the molecules.
*See also
Allotropes of phosphorus are useful to further demonstrate this structure-property relationship. White phosphorus, a molecular solid, has a relatively low density of 1.82 g/cm3 and melting point of 44.1 °C; it is a soft material which can be cut with a knife. When it is converted to the covalent red phosphorus, the density goes to 2.2–2.4 g/cm3 and melting point to 590 °C, and when white phosphorus is transformed into the (also covalent) black phosphorus, the density becomes 2.69–3.8 g/cm3 and melting temperature ~200 °C. Both red and black phosphorus forms are significantly harder than white phosphorus.
higher alkanes
Higher alkanes are alkanes having nine or more carbon atoms. Nonane is the lightest alkane to have a flash point above 25 °C, and is not classified as dangerously flammable.
The term ''higher alkanes'' is sometimes used literally as "alkanes ...
Mechanical properties
Molecular solids can be either ductile or brittle, or a combination depending on thecrystal face
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macrosc ...
stressed. Both ductile and brittle solids undergo elastic deformation till they reach the yield stress. Once the yield stress is reached ductile solids undergo a period of plastic deformation, and eventually fracture. Brittle solids fracture promptly after passing the yield stress. Due to the asymmetric structure of most molecules, many molecular solids have directional intermolecular forces. This phenomenon can lead to anisotropic mechanical properties. Typically a molecular solid is ductile when it has directional intermolecular interactions. This allows for dislocation between layers of the crystal much like metals.
One example of a ductile molecular solid, that can be bent 180°, is hexachlorobenzene (HCB). In this example the π-π interactions between the benzene cores are stronger than the halogen interactions of the chlorides. This difference leads to its flexibility. This flexibility is anisotropic; to bend HCB to 180° you must stress the 01face of the crystal. Another example of a flexible molecular solid is 2-(methylthio)nicotinic acid (MTN). MTN is flexible due to its strong hydrogen bonding and π-π interactions creating a rigid set of dimers that dislocate along the alignment of their terminal methyls. When stressed on the 10face this crystal will bend 180°. Note, not all ductile molecular solids bend 180° and some may have more than one bending faces.
Electrical properties
Molecular solids are generally insulators. This large band gap (compared togermanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors s ...
at 0.7 eV) is due to the weak intermolecular interactions, which result in low charge carrier mobility
Charge or charged may refer to:
Arts, entertainment, and media Films
* ''Charge, Zero Emissions/Maximum Speed'', a 2011 documentary
Music
* ''Charge'' (David Ford album)
* ''Charge'' (Machel Montano album)
* ''Charge!!'', an album by The Aqua ...
. Some molecular solids exhibit electrical conductivity, such as TTF-TCNQ
Tetrathiafulvalene is an organosulfur compound with the formula (. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, , by replacement of four CH groups wit ...
with ρ = 5 x 102 Ω−1 cm−1 but in such cases orbital overlap is evident in the crystal structure. Fullerenes, which are insulating, become conducting or even superconducting upon doping.
Thermal properties
Molecular solids have many thermal properties: specific heat capacity, thermal expansion, and thermal conductance to name a few. These thermal properties are determined by the intra- and intermolecular vibrations of the atoms and molecules of the molecular solid. While transitions of an electron do contribute to thermal properties, their contribution is negligible compared to the vibrational contribution.See also
* Bonding in solidsReferences
* https://www.boundless.com/chemistry/liquids-and-solids/types-of-crystals/molecular-crystals/ {{DEFAULTSORT:Molecular Solid Chemical compounds