|
Pi Interaction
In chemistry, π-effects or π-interactions are a type of non-covalent interaction that involves π systems. Just like in an electrostatic interaction where a region of negative charge interacts with a positive charge, the electron-rich π system can interact with a metal (cationic or neutral), an anion, another molecule and even another π system. Non-covalent interactions involving π systems are pivotal to biological events such as protein-ligand recognition. Types The most common types of π-interactions involve: *Metal–π interactions: involves interaction of a metal and the face of a π system, the metal can be a cation (known as cation–π interactions) or neutral *Polar–π interactions: involves interaction of a polar molecule and quadrupole moment a π system. * Aromatic–aromatic interactions (π stacking): involves interactions of aromatic molecules with each other. **Arene–perfluoroarene interaction: electron-rich benzene ring interacts with electron-poor hex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pi Bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals has an electron density of zero at a shared nodal plane that passes through the two bonded nuclei. This plane also is a nodal plane for the molecular orbital of the pi bond. Pi bonds can form in double and triple bonds but do not form in single bonds in most cases. The Greek letter π in their name refers to p orbitals, since the orbital symmetry of the pi bond is the same as that of the p orbital when seen down the bond axis. One common form of this sort of bonding involves p orbitals themselves, though d orbitals also engage in pi bonding. This latter mode forms part of the basis for metal-metal multiple bonding. Pi bonds are usually weaker than sigma bonds. The C-C double bond, composed of one sigma and one pi bond, ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Petasis Reaction
The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl-boronic acid to form substituted amines. Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine. In the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery. Reaction scope and synthetic applications The amine is condensed with the carbonyl followed by addition of the boronic acid . ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Negishi Coupling
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process. A palladium (0) species is generally utilized as the metal catalyst, though nickel is sometimes used. A variety of nickel catalysts in either Ni0 or NiII oxidation state can be employed in Negishi cross couplings such as Ni(PPh3)4, Ni(acac)2, Ni(COD)2 etc. : :* The leaving group X is usually chloride, bromide, or iodide, but triflate and acetyloxy groups are feasible as well. X = Cl usually leads to slow reactions. :* The organic residue R = alkenyl, aryl, allyl, alkynyl or propargyl. :* The halide X' in the organozinc compound can be chloride, bromine or iodine and the organic residue R' is alkenyl, aryl, allyl, alkyl, benzyl, homoallyl, and homopropargyl. :* The metal M in the catalyst is nickel or palladium :* The ligand L in the catalyst can be t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kumada Coupling
In organic chemistry, the Kumada coupling is a type of cross coupling reaction, useful for generating carbon–carbon bonds by the reaction of a Grignard reagent and an organic halide. The procedure uses transition metal catalysts, typically nickel or palladium, to couple a combination of two alkyl, aryl or vinyl groups. The groups of Robert Corriu and Makoto Kumada reported the reaction independently in 1972. The reaction is notable for being among the first reported catalytic cross-coupling methods. Despite the subsequent development of alternative reactions (Suzuki, Sonogashira, Stille, Hiyama, Negishi), the Kumada coupling continues to be employed in many synthetic applications, including the industrial-scale production of aliskiren, a hypertension medication, and polythiophenes, useful in organic electronic devices. History The first investigations into the catalytic coupling of Grignard reagents with organic halides date back to the 1941 study of cobalt catalyst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hiyama Coupling
The Hiyama coupling is a palladium-catalyzed cross-coupling reaction of organosilanes with organic halides used in organic chemistry to form carbon–carbon bonds (C-C bonds). This reaction was discovered in 1988 by Tamejiro Hiyama and Yasuo Hatanaka as a method to form carbon-carbon bonds synthetically with chemo- and regioselectivity. The Hiyama coupling has been applied to the synthesis of various natural products. :\begin\\ \ce \end :* R: aryl, alkenyl or alkynyl :* R': aryl, alkenyl, alkynyl or alkyl :* R'': Cl, F or alkyl :* X: Cl, Br, I or OTf Reaction history The Hiyama coupling was developed to combat the issues associated with other organometallic reagents. The initial reactivity of organosilicon was not actually first reported by Hiyama, as Kumada reported a coupling reaction using organofluorosilicates shown below. Organosilanes were then discovered, by Hiyama, to have reactivity when activated by a fluoride source. This reactivity, when combined with a palladium sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heck Reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a substituted alkene. It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The Heck reaction is a way to substitute alkenes. History The original reaction by Tsutomu Mizoroki (1971) describes the coupling between iodobenzene and styrene in methanol to form stilbene at 120 °C (autoclave) with potassium acetate base and palladium chloride catalysis. This work was a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
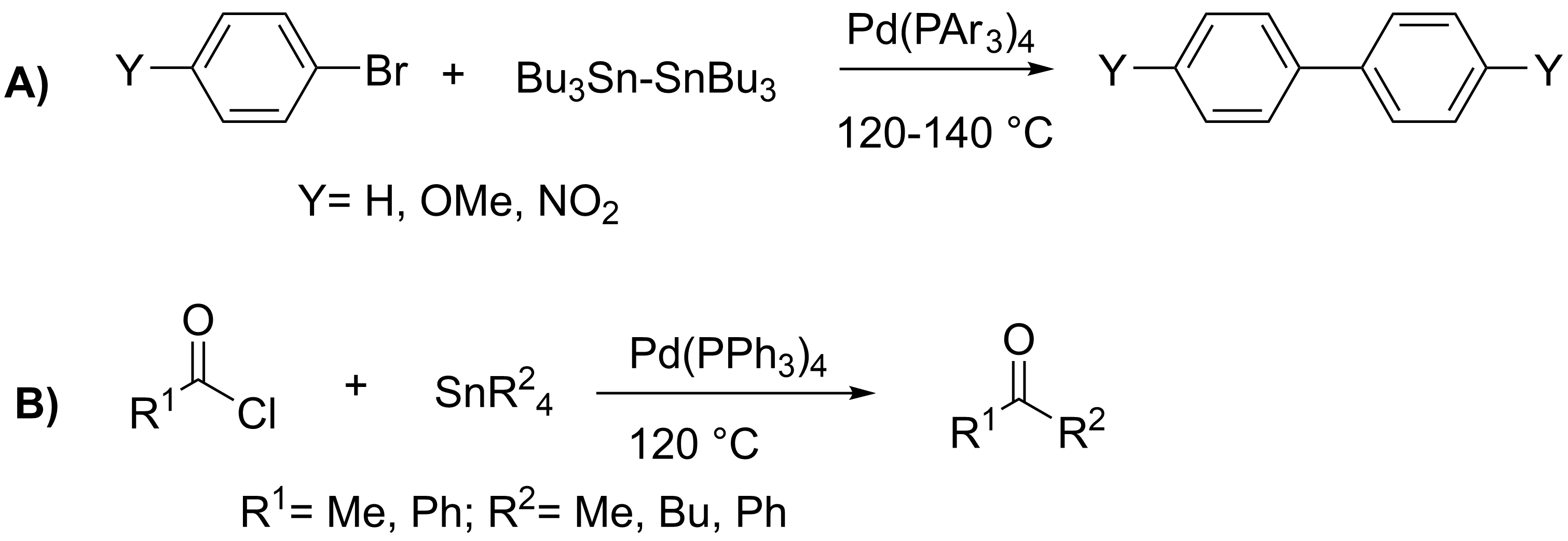
Stille Reaction
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin compound (also known as organostannanes). A variety of organic electrophiles provide the other coupling partner. The Stille reaction is one of many palladium-catalyzed coupling reactions.Hartwig, J. F. ''Organotransition Metal Chemistry, from Bonding to Catalysis''; University Science Books: New York, 2010. Stille, J. K. ''Angew. Chem. Int. Ed. Engl.'' 1986, ''25'', 508–524.ReviewFarina, V.; Krishnamurthy, V.; Scott, W. J. ''Org. React.'' 1998, ''50'', 1–652.Review : + \ \ce \ \overbrace^ + \!-\! :*\!,\ : Allyl, alkenyl, aryl, benzyl,acyl :*: halides (Cl, Br, I), pseudohalides (, OPO(OR)2), OAc The R1 group attached to the trialkyltin is normally sp2-hybridized, including vinyl, and aryl groups. These organostannanes are also stable to both air and moisture, and many of these reagents either are comme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels-Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic chemistry has a strong tradition of naming a specific reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienyl Complex
A cyclopentadienyl complex is a coordination complex of a metal and cyclopentadienyl groups (, abbreviated as Cp−). Cyclopentadienyl ligands almost invariably bind to metals as a pentahapto (''η''5-) bonding mode. The metal–cyclopentadienyl interaction is typically drawn as a single line from the metal center to the center of the Cp ring.Elschenbroich, C. "Organometallics" (2006) Wiley-VCH: Weinheim. Examples ''Bis''cyclopentadienyl complexes are called metallocenes. A famous example of this type of complex is ferrocene (FeCp2), which has many analogues for other metals, such as chromocene (CrCp2), cobaltocene (CoCp2), and nickelocene (NiCp2). When the Cp rings are mutually parallel the compound is known as a sandwich complex. This area of organometallic chemistry was first developed in the 1950s. Bent metallocenes are represented by compounds of the type Cp2Lx Some are catalysts for ethylene polymerization. Metallocenes are often thermally stable, and find use a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |





2.png)