LSM mode on:
[Wikipedia]
[Google]
[Amazon]
 In molecular biology, LSm proteins are a family of
In molecular biology, LSm proteins are a family of
 Uridine phosphate binds in archaeal Sm1 between the β2b/β3a loop and β4b/β5 loop. The uracil is stacked between the histidine and
Uridine phosphate binds in archaeal Sm1 between the β2b/β3a loop and β4b/β5 loop. The uracil is stacked between the histidine and
Pfam entry LSM. Pfam is the Sanger Institute database, which is a collection of protein families and domains.
{{DEFAULTSORT:Lsm Protein families Spliceosome
RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
-binding proteins found in virtually every cellular organism. LSm is a contraction of 'like Sm', because the first identified members of the LSm protein family
A protein family is a group of evolutionarily related proteins. In many cases, a protein family has a corresponding gene family, in which each gene encodes a corresponding protein with a 1:1 relationship. The term "protein family" should not be c ...
were the Sm proteins. LSm proteins are defined by a characteristic three-dimensional structure and their assembly into rings of six or seven individual LSm protein molecules, and play a large number of various roles in mRNA processing and regulation.
The Sm proteins were first discovered as antigens
In immunology, an antigen (Ag) is a molecule or molecular structure or any foreign particulate matter or a pollen grain that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response. ...
targeted by so-called anti-Sm antibodies in a patient with a form of systemic lupus erythematosus
Lupus, technically known as systemic lupus erythematosus (SLE), is an autoimmune disease in which the body's immune system mistakenly attacks healthy tissue in many parts of the body. Symptoms vary among people and may be mild to severe. Comm ...
(SLE), a debilitating autoimmune disease
An autoimmune disease is a condition arising from an abnormal immune response to a functioning body part. At least 80 types of autoimmune diseases have been identified, with some evidence suggesting that there may be more than 100 types. Nearly a ...
. They were named Sm proteins in honor of Stephanie Smith, a patient who suffered from SLE. Other proteins with very similar structures were subsequently discovered and named LSm proteins. New members of the LSm protein family continue to be identified and reported.
Proteins with similar structures are grouped into a hierarchy of protein families, superfamilies, and folds. The LSm protein structure is an example of a small beta sheet
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a g ...
folded into a short barrel. Individual LSm proteins assemble into a six or seven member doughnut ring (more properly termed a torus), which usually binds to a small RNA
Small RNA (sRNA) are polymeric RNA molecules that are less than 200 nucleotides in length, and are usually non-coding
Non-coding DNA (ncDNA) sequences are components of an organism's DNA that do not encode protein sequences. Some non-coding DNA ...
molecule to form a ribonucleoprotein complex. The LSm torus assists the RNA molecule to assume and maintain its proper three-dimensional structure. Depending on which LSm proteins and RNA molecule are involved, this ribonucleoprotein complex facilitates a wide variety of RNA processing including degradation, editing, splicing, and regulation.
Alternate terms for LSm family are LSm fold and Sm-like fold, and alternate capitalization styles such as lsm, LSM, and Lsm are common and equally acceptable.
History
Discovery of the Smith antigen
The story of the discovery of the first LSm proteins begins with a young woman, Stephanie Smith, who was diagnosed in 1959 withsystemic lupus erythematosus (SLE)
Lupus, technically known as systemic lupus erythematosus (SLE), is an autoimmune disease in which the body's immune system mistakenly attacks healthy tissue in many parts of the body. Symptoms vary among people and may be mild to severe. Comm ...
, eventually succumbing to complications of the disease in 1969 at the age of 22. During this period, she was treated at New York's Rockefeller University
The Rockefeller University is a private biomedical research and graduate-only university in New York City, New York. It focuses primarily on the biological and medical sciences and provides doctoral and postdoctoral education. It is classif ...
Hospital, under the care of Dr. Henry Kunkel and Dr. Eng Tan. As those with an autoimmune disease
An autoimmune disease is a condition arising from an abnormal immune response to a functioning body part. At least 80 types of autoimmune diseases have been identified, with some evidence suggesting that there may be more than 100 types. Nearly a ...
, SLE patients produce antibodies
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the ...
to antigens in their cells' nuclei, most frequently to their own DNA. However, Dr. Kunkel and Dr. Tan found in 1966 that Ms. Smith produced antibodies
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the ...
to a set of nuclear proteins, which they named the 'smith antigen' (Sm Ag). About 30% of SLE patients produce antibodies to these proteins, as opposed to double stranded DNA. This discovery improved diagnostic testing for SLE, but the nature and function of this antigen was unknown.
Sm proteins, snRNPs, the spliceosome and messenger RNA splicing
Research continued during the 1970s and early 1980s. The smith antigen was found to be a complex of ribonucleic acid (RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
) molecules and multiple proteins. A set of uridine-rich small nuclear RNA (snRNA) molecules was part of this complex, and given the names U1, U2, U4, U5 and U6. Four of these snRNAs (U1, U2, U4 and U5) were found to be tightly bound to several small proteins, which were named SmB, SmD, SmE, SmF, and SmG in decreasing order of size. SmB has an alternatively spliced variant, SmB', and a very similar protein, SmN, replaces SmB'/B in certain (mostly neural) tissues. SmD was later discovered to be a mixture of three proteins, which were named SmD1, SmD2 and SmD3. These nine proteins (SmB, SmB', SmN, SmD1, SmD2, SmD3, SmE, SmF and SmG) became known as the Sm core proteins, or simply Sm proteins. The snRNAs are complexed with the Sm core proteins and with other proteins to form particles in the cell's nucleus called small nuclear ribonucleoproteins, or snRNPs. By the mid 1980s, it became clear that these snRNPs help form a large (4.8 MD molecular weight) complex, called the spliceosome, around pre-mRNA, excising portions of the pre-mRNA called intron
An intron is any nucleotide sequence within a gene that is not expressed or operative in the final RNA product. The word ''intron'' is derived from the term ''intragenic region'', i.e. a region inside a gene."The notion of the cistron .e., gene. ...
s and splicing the coding portions (exon
An exon is any part of a gene that will form a part of the final mature RNA produced by that gene after introns have been removed by RNA splicing. The term ''exon'' refers to both the DNA sequence within a gene and to the corresponding sequen ...
s) together. After a few more modifications, the spliced pre-mRNA becomes messenger RNA
In molecular biology, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and is read by a ribosome in the process of synthesizing a protein.
mRNA is created during the p ...
(mRNA) which is then exported from the nucleus and translated
Translation is the communication of the meaning of a source-language text by means of an equivalent target-language text. The English language draws a terminological distinction (which does not exist in every language) between ''transla ...
into a protein by ribosomes
Ribosomes ( ) are macromolecular machines, found within all cells, that perform biological protein synthesis (mRNA translation). Ribosomes link amino acids together in the order specified by the codons of messenger RNA (mRNA) molecules to f ...
.
Discovery of proteins similar to the Sm proteins
The snRNA U6 (unlike U1, U2, U4 and U5) does not associate with the Sm proteins, even though the U6 snRNP is a central component in the spliceosome. In 1999 a protein heteromer was found that binds specifically to U6, and consisted of seven proteins clearly homologous to the Sm proteins. These proteins were denoted LSm (like Sm) proteins (LSm1, LSm2, LSm3, LSm4, LSm5, LSm6 and LSm7), with the similar LSm8 protein identified later. In the bacterium '' Escherichia coli'', the Sm-like protein HF-I encoded by the gene ''hfq
The Hfq protein (also known as HF-I protein) encoded by the ''hfq'' gene was discovered in 1968 as an ''Escherichia coli'' host factor that was essential for replication of the bacteriophage Qβ. It is now clear that Hfq is an abundant bacterial RN ...
'' was described in 1968 as an essential host factor for RNA bacteriophage
A bacteriophage (), also known informally as a ''phage'' (), is a duplodnaviria virus that infects and replicates within bacteria and archaea. The term was derived from "bacteria" and the Greek φαγεῖν ('), meaning "to devour". Bacteri ...
Qβ replication. The genome of '' Saccharomyces cerevisiae'' (Baker's Yeast) was sequenced in the mid-1990s, providing a rich resource for identifying homologs of these human proteins. Subsequently, as more eukaryote
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
s genomes were sequenced, it became clear that eukaryotes, in general, share homologs to the same set of seven Sm and eight LSm proteins. Soon after, proteins homologous to these eukaryote LSm proteins were found in Archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebac ...
(Sm1 and Sm2) and Bacteria (Hfq and YlxS homologs). The archaeal LSm proteins are more similar to the eukaryote LSm proteins than either are to bacterial LSm proteins. The LSm proteins described thus far were rather small proteins, varying from 76 amino acids (8.7 kD molecular weight) for human SmG to 231 amino acids (29 kD molecular weight) for human SmB. But recently, larger proteins have been discovered that include a LSm structural domain in addition to other protein structural domains (such as LSm10, LSm11, LSm12, LSm13, LSm14, LSm15, LSm16, ataxin-2, as well as archaeal Sm3).
Discovery of the LSm fold
Around 1995, comparisons between the various LSm homologs identified twosequence motif
In biology, a sequence motif is a nucleotide or amino-acid sequence pattern that is widespread and usually assumed to be related to biological function of the macromolecule. For example, an ''N''-glycosylation site motif can be defined as ''As ...
s, 32 nucleic acids long (14 amino acids), that were very similar in each LSm homolog, and were separated by a non-conserved region of variable length. This indicated the importance of these two sequence motifs (named Sm1 and Sm2), and suggested that all LSm protein genes evolved from a single ancestral gene. In 1999, crystals of recombinant Sm proteins were prepared, allowing X-ray crystallography and determination of their atomic structure in three dimensions. This demonstrated that the LSm proteins share a similar three-dimensional fold
Fold, folding or foldable may refer to:
Arts, entertainment, and media
* ''Fold'' (album), the debut release by Australian rock band Epicure
*Fold (poker), in the game of poker, to discard one's hand and forfeit interest in the current pot
*Above ...
of a short alpha helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues e ...
and a five-stranded folded beta sheet
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a g ...
, subsequently named the LSm fold. Other investigations found that LSm proteins assemble into a torus (doughnut-shaped ring) of six or seven LSm proteins, and that RNA binds to the inside of the torus, with one nucleotide bound to each LSm protein.
Structure
arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the am ...
residues, stabilized by hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ing to an asparagine residue, and hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ing between the aspartate
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the pro ...
residue and the ribose. LSm proteins are characterized by a beta sheet
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a g ...
(the secondary structure
Protein secondary structure is the three dimensional conformational isomerism, form of ''local segments'' of proteins. The two most common Protein structure#Secondary structure, secondary structural elements are alpha helix, alpha helices and beta ...
), folded into the LSm fold (the tertiary structure), polymerization into a six or seven member torus (the quaternary structure), and binding to RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
oligonucleotides. A modern paradigm classifies proteins on the basis of protein structure and is a currently active field, with three major approaches, SCOP (Structural Classification of Proteins), CATH
The CATH Protein Structure Classification database is a free, publicly available online resource that provides information on the evolutionary relationships of protein domains. It was created in the mid-1990s by Professor Christine Orengo and coll ...
(Class, Architecture, Topology, Homologous superfamily), and FSSP/DALI (Families of Structurally Similar Proteins).
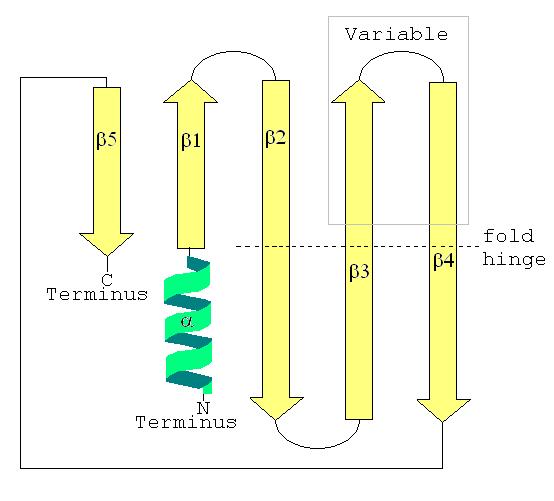
Secondary
Thesecondary structure
Protein secondary structure is the three dimensional conformational isomerism, form of ''local segments'' of proteins. The two most common Protein structure#Secondary structure, secondary structural elements are alpha helix, alpha helices and beta ...
of a LSm protein is a small five-strand anti-parallel beta sheet
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a g ...
, with the strands identified from the N-terminal end
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amin ...
to the C-terminal end as β1, β2, β3, β4, β5. The SCOP class of All beta proteins and the CATH class of Mainly Beta are defined as protein structures that are primarily beta sheets, thus including LSm. The SM1 sequence motif
In biology, a sequence motif is a nucleotide or amino-acid sequence pattern that is widespread and usually assumed to be related to biological function of the macromolecule. For example, an ''N''-glycosylation site motif can be defined as ''As ...
corresponds to the β1, β2, β3 strands, and the SM2 sequence motif corresponds to the β4 and β5 strands. The first four beta strands are adjacent to each other, but β5 is adjacent to β1, turning the overall structure into a short barrel. This structural topology is described as 51234. A short (two to four turns) N-terminal alpha helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues e ...
is also present in most LSm proteins. The β3 and β4 strands are short in some LSm proteins, and are separated by an unstructured coil of variable length. The β2, β3 and β4 strands are strongly bent about 120° degrees at their midpoints The bends in these strands are often glycine, and the side chains internal to the beta barrel are often the hydrophobic residues valine, leucine, isoleucine
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the deprot ...
and methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical ro ...
.
Tertiary
SCOP simply classifies the LSm structure as the Sm-like fold, one of 149 different Beta Protein folds, without any intermediate groupings. The LSm beta sheet is sharply bent and described as a Roll architecture in CATH (one of 20 different beta protein architectures in CATH). One of the beta strands (β5 in LSm) crosses the open edge of the roll to form a small SH3 type barrel topology (one of 33 beta roll topologies in CATH). CATH lists 23 homologous superfamilies with an SH3 type barrel topology, one of which is the LSm structure (RNA Binding Protein in the CATH system). SCOP continues its structural classification after Fold to Superfamily, Family and Domain, while CATH continues to Sequence Family, but these divisions are more appropriately described in the "Evolution and phylogeny" section. The SH3-type barrel tertiary structure of the LSm fold is formed by the strongly bent (about 120°) β2, β3 and β4 strands, with the barrel structure closed by the β5 strand. Emphasizing the tertiary structure, each bent beta strand can be described as two shorter beta strands. The LSm fold can be viewed as an eight-strand anti-parallel beta sandwich, with five strands in one plane and three strands in a parallel plane with about a 45° pitch angle between the two halves of the beta sandwich. The short (two to four turns) N-terminalalpha helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues e ...
occurs at one edge of the beta sandwich. This alpha helix and the beta strands can be labeled (from the N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the ami ...
to the C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is ...
) α, β1, β2a, β2b, β3a, β3b, β4a, β4b, β5 where the a and b refer to either the two halves of a bent strand in the five-strand description, or to the individual strands in the eight-strand description. Each strand (in the eight-strand description) is formed from five amino acid residues. Including the bends and loops between the strands, and the alpha helix, about 60 amino acid residues contribute to the LSm fold, but this varies between homologs due to variation in inter-strand loops, the alpha helix, and even the lengths of β3b and β4a strands.
Quaternary
LSm proteins typically assemble into a LSm ring, a six or seven member torus, about 7 nanometers in diameter with a 2 nanometer hole. The ancestral condition is a homohexamer or homoheptamer of identical LSm subunits. LSm proteins ineukaryote
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
s form heteroheptamers of seven different LSm subunits, such as the Sm proteins. Binding between the LSm proteins is best understood with the eight-strand description of the LSm fold. The five-strand half of the beta sandwich of one subunit aligns with the three-strand half of the beta sandwich of the adjacent subunit, forming a twisted 8-strand beta sheet Aβ4a/Aβ3b/Aβ2a/Aβ1/Aβ5/Bβ4b/Bβ3a/Bβ2b, where the A and B refer to the two different subunits. In addition to hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ing between the Aβ5 and Bβ4b beta strands of the two LSm protein subunits, there are energetically favorable contacts between hydrophobic amino acid side chains in the interior of the contact area, and energetically favorable contacts between hydrophilic amino acid side chains around the periphery of the contact area.
RNA oligonucleotide binding
LSm rings form ribonucleoprotein complexes withRNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
oligonucleotides that vary in binding strength from very stable complexes (such as the Sm class snRNPs) to transient complexes. RNA oligonucleotides generally bind inside the hole (lumen) of the LSm torus, one nucleotide per LSm subunit, but additional nucleotide binding sites have been reported at the top (α helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues ...
side) of the ring. The exact chemical nature of this binding varies, but common motifs include stacking the heterocyclic base (often uracil) between planar side chains of two amino acids, hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ing to the heterocyclic base and/or the ribose, and salt bridges to the phosphate group.
Functions
The various kinds of LSm rings function as scaffolds or chaperones forRNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
oligonucleotides, assisting the RNA to assume and maintain the proper three-dimensional structure. In some cases, this allows the oligonucleotide RNA to function catalytically as a ribozyme. In other cases, this facilitates modification or degradation of the RNA, or the assembly, storage, and intracellular transport of ribonucleoprotein complexes.
Sm ring
The Sm ring is found in thenucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucle ...
of all eukaryote
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
s (about 2.5 x 106 copies per proliferating human cell), and has the best understood functions. The Sm ring is a heteroheptamer. The Sm-class snRNA molecule (in the 5' to 3' direction) enters the lumen (doughnut hole) at the SmE subunit and proceeds sequentially in a clockwise fashion (looking from the α helix side) inside the lumen (doughnut hole) to the SmG, SmD3, SmB, SmD1, SmD2 subunits, exiting at the SmF subunit. (SmB can be replaced by the splice variant SmB' and by SmN in neural tissues.) The Sm ring permanently binds to the U1, U2, U4 and U5 snRNAs which form four of the five snRNPs that constitute the major spliceosome. The Sm ring also permanently binds to the U11, U12 and U4atac snRNAs which form four of the five snRNPs (including the U5 snRNP) that constitute the minor spliceosome The minor spliceosome is a ribonucleoprotein complex that catalyses the removal ( splicing) of an atypical class of spliceosomal introns (U12-type) from messenger RNAs in some clades of eukaryotes. This process is called noncanonical splicing, as op ...
. Both of these spliceosomes are central RNA-processing complexes in the maturation of messenger RNA
In molecular biology, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and is read by a ribosome in the process of synthesizing a protein.
mRNA is created during the p ...
from pre-mRNA. Sm proteins have also been reported to be part of ribonucleoprotein component of telomerase.
Lsm2-8 ring
The two Lsm2-8 snRNPs (U6 and U6atac) have the key catalytic function in the major and minor spliceosomes. These snRNPs do not include the Sm ring, but instead use the heteroheptameric Lsm2-8 ring. The LSm rings are about 20 times less abundant than the Sm rings. The order of these seven LSm proteins in this ring is not known, but based on amino acid sequence homology with the Sm proteins, it is speculated that the snRNA (in the 5' to 3' direction) may bind first to LSm5, and precedes sequentially clockwise to the LSm7, LSm4, LSm8, LSm2, LSm3, and exiting at the LSm6 subunit. Experiments with '' Saccharomyces cerevisiae'' (budding yeast) mutations suggest that the Lsm2-8 ring assists the reassociation of the U4 and U6 snRNPs into the U4/U6 di-snRNP. (After completion of exon deletion and intron splicing, these two snRNPs must reassociate for the spliceosome to initiate another exon/intron splicing cycle. In this role, the Lsm2-8 ring acts as an RNA chaperone instead of an RNA scaffold.) The Lsm2-8 ring also forms an snRNP with the U8small nucleolar RNA
In molecular biology, Small nucleolar RNAs (snoRNAs) are a class of small RNA molecules that primarily guide chemical modifications of other RNAs, mainly ribosomal RNAs, transfer RNAs and small nuclear RNAs. There are two main classes of snoRNA, t ...
(snoRNA) which localizes in the nucleolus. This ribonucleoprotein complex is necessary for processing ribosomal RNA and transfer RNA
Transfer RNA (abbreviated tRNA and formerly referred to as sRNA, for soluble RNA) is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes), that serves as the physical link between the mRNA and the amino ac ...
to their mature forms. The Lsm2-8 ring is reported to have a role in the processing of pre-P RNA into RNase P RNA. In contrast to the Sm ring, the Lsm2-8 ring does not permanently bind to its snRNA and snoRNA.
Sm10/Sm11 ring
A second type of Sm ring exists whereLSm10
U7 snRNA-associated Sm-like protein LSm10 is a protein that in humans is encoded by the ''LSM10'' gene.
Interactions
LSM10 has been shown to interact
Advocates for Informed Choice, doing business as, dba interACT or interACT Advocates for ...
replaces SmD1 and LSm11 replaces SmD2. LSm11 is a two domain protein with the C-terminal domain being a LSm domain. This heteroheptamer ring binds with the U7 snRNA in the U7 snRNP. The U7 snRNP mediates processing of the 3' UTR stem-loop of the histone mRNA in the nucleus. Like the Sm ring, it is assembled in the cytoplasm onto the U7 snRNA by a specialized SMN complex.
Lsm1-7 ring
A second type of Lsm ring is the Lsm1-7 ring, which has the same structure as the Lsm2-8 ring except that LSm1 replaces LSm8. In contrast to the Lsm2-8 ring, the Lsm1-7 ring localizes in the cytoplasm where it assists in degradingmessenger RNA
In molecular biology, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and is read by a ribosome in the process of synthesizing a protein.
mRNA is created during the p ...
in ribonucleoprotein complexes. This process controls the turnover of messenger RNA so that ribosomal translation of mRNA to protein responds quickly to changes in transcription
Transcription refers to the process of converting sounds (voice, music etc.) into letters or musical notes, or producing a copy of something in another medium, including:
Genetics
* Transcription (biology), the copying of DNA into RNA, the fir ...
of DNA to messenger RNA by the cell. LSM1-7, together with Pat1, has been shown to play a role in the formation of P-bodies after deadenylation.
Gemin6 and Gemin7
The SMN complex (described under "Biogenesis of snRNPs") is composed of the SMN protein and Gemin2-8. Two of these, Gemin 6 and Gemin7 have been discovered to have the LSm structure, and to form a heterodimer. These may have a chaperone function in the SMN complex to assist the formation of the Sm ring on the Sm-class snRNAs. PRMT5 complex is composed ofPRMT5
Protein arginine N-methyltransferase 5 is an enzyme that in humans is encoded by the ''PRMT5'' gene. PRMT5 symmetrically dimethylates H2AR3, H4R3, H3R2, and H3R8 in vivo, all of which are linked to a range of transcriptional regulatory events.
PR ...
, pICln, WD45 (Mep50). pICln helps to form Sm opened ring on SMN complex. SMN complex assists in the assembly of snRNPs where the Sm ring is in the open conformation on SMN complex and this Sm ring is loaded onto the snRNA by SMN complex.
LSm12-16 and other multi-domain LSm proteins
The LSm12-16 proteins have been described very recently. These are two-domain proteins with aN-terminal
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the ami ...
LSm domain and a C-terminal methyl transferase domain. Very little is known about the function of these proteins, but presumably they are member of LSm-domain rings that interact with RNA. There is some evidence that LSm12 is possibly involved in mRNA degradation and LSm13-16 may have roles in regulation of mitosis
In cell biology, mitosis () is a part of the cell cycle in which replicated chromosomes are separated into two new nuclei. Cell division by mitosis gives rise to genetically identical cells in which the total number of chromosomes is mainta ...
. Unexpectedly, LSm12 was recently implicated in Calcium signaling by acting as the intermediate binding-protein for the nucleotide second messenger, NAADP (Nicotinic acid adenine dinucleotide phosphate
Nicotinic acid adenine dinucleotide phosphate, (NAADP), is a Ca2+-mobilizing second messenger synthesised in response to extracellular stimuli. Like its mechanistic cousins, IP3 and cyclic adenosine diphosphoribose (Cyclic ADP-ribose), NAADP bind ...
) that activates endo-lysosomal Ca2+ channels TPCs (Two-pore channel
Two-pore channels (TPCs) are eukaryotic intracellular voltage-gated and ligand gated cation selective ion channels. There are two known paralogs in the human genome, TPC1s and TPC2s. In humans, TPC1s are sodium selective and TPC2s conduct so ...
s). This occurred by NAADP binding to the LSm domain, not the AD domain. A large protein of unknown function, ataxin-2, associated with the neurodegenerative disease spinocerebellar ataxia type 2, also has a N-terminal LSm domain.
Archaeal Sm rings
Two LSm proteins are found in a seconddomain
Domain may refer to:
Mathematics
*Domain of a function, the set of input values for which the (total) function is defined
**Domain of definition of a partial function
**Natural domain of a partial function
**Domain of holomorphy of a function
* Do ...
of life, the Archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebac ...
. These are the Sm1 and Sm2 proteins (not to be confused with the Sm1 and Sm2 sequence motif
In biology, a sequence motif is a nucleotide or amino-acid sequence pattern that is widespread and usually assumed to be related to biological function of the macromolecule. For example, an ''N''-glycosylation site motif can be defined as ''As ...
s), and are sometimes identified as Sm-like archaeal proteins SmAP1 and SmAP2 for this reason. Sm1 and Sm2 generally form homoheptamer rings, although homohexamer rings have been observed. Sm1 rings are similar to eukaryote
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
Lsm rings in that they form in the absence of RNA while Sm2 rings are similar to eukaryote
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
Sm rings in that they require uridine-rich RNA for their formation. They have been reported to associate with RNase P RNA, suggesting a role in transfer RNA
Transfer RNA (abbreviated tRNA and formerly referred to as sRNA, for soluble RNA) is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes), that serves as the physical link between the mRNA and the amino ac ...
processing, but their function in archaea in this process (and possibly processing other RNA such as ribosomal RNA) is mostly unknown. One of the two main branches of archaea, the crenarchaeotes have a third known type of archaeal LSm protein, Sm3. This is a two-domain protein with a N-terminal
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the ami ...
LSm domain that forms a homoheptamer ring. Nothing is known about the function of this LSm protein, but presumably it interacts with, and probably helps process, RNA in these organisms.
Bacterial LSm rings
Several LSm proteins have been reported in the thirddomain
Domain may refer to:
Mathematics
*Domain of a function, the set of input values for which the (total) function is defined
**Domain of definition of a partial function
**Natural domain of a partial function
**Domain of holomorphy of a function
* Do ...
of life, the Bacteria. Hfq protein forms homohexamer rings, and was originally discovered as necessary for infection by the bacteriophage Qβ, although this is clearly not the native function of this protein in bacteria. It is not universally present in all bacteria, but has been found in Pseudomonadota
Pseudomonadota (synonym Proteobacteria) is a major phylum of Gram-negative bacteria. The renaming of phyla in 2021 remains controversial among microbiologists, many of whom continue to use the earlier names of long standing in the literature. The ...
, Bacillota, Spirochaetota
A spirochaete () or spirochete is a member of the phylum Spirochaetota (), (synonym Spirochaetes) which contains distinctive diderm (double-membrane) gram-negative bacteria, most of which have long, helically coiled (corkscrew-shaped or ...
, Thermotogota, Aquificota, and one species of Archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebac ...
. (This last instance is probably a case of horizontal gene transfer.) Hfq is pleiotropic with a variety of interactions, generally associated with translation regulation. These include blocking ribosome binding to mRNA, marking mRNA for degradation by binding to their poly-A tails, and association with bacterial small regulatory RNAs (such as DsrA RNA) that control translation by binding to certain mRNAs
In molecular biology, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and is read by a ribosome in the process of synthesizing a protein.
mRNA is created during the p ...
. A second bacterial LSm protein is YlxS (sometimes also called YhbC), which was first identified in the soil bacterium '' Bacillus subtilis''. This is a two-domain protein with a N-terminal
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the ami ...
LSm domain. Its function is unknown, but amino acid sequence homologs are found in virtually every bacterial genome to date, and it may be an essential protein. The middle domain of the small conductance mechanosensitive channel MscS in '' Escherichia coli'' forms a homoheptameric ring. This LSm domain has no apparent RNA-binding function, but the homoheptameric torus is part of the central channel of this membrane protein.
Evolution and phylogeny
LSm homologs are found in all three domains of life, and may even be found in every single organism.Computational phylogenetic
Computational phylogenetics is the application of computational algorithms, methods, and programs to phylogenetic
methods are used to infer Phylogenetics, phylogenetic relations. Sequence alignment between the various LSm homologs are the appropriate tool for this, such as multiple sequence alignment of the primary structure (amino acid sequence), and structural alignment
Structural alignment attempts to establish homology between two or more polymer structures based on their shape and three-dimensional conformation. This process is usually applied to protein tertiary structures but can also be used for large RN ...
of the tertiary structure (three-dimensional structure). It is hypothesized that a gene for a LSm protein was present in the last universal ancestor of all life. Based on the functions of known LSm proteins, this original LSm protein may have assisted ribozymes in the processing of RNA for synthesizing proteins as part of the RNA world hypothesis of early life. According to this view, this gene was passed from ancestor to descendant, with frequent mutations, gene duplication
Gene duplication (or chromosomal duplication or gene amplification) is a major mechanism through which new genetic material is generated during molecular evolution. It can be defined as any duplication of a region of DNA that contains a gene. ...
s and occasional horizontal gene transfers. In principle, this process can be summarized in a phylogenetic tree
A phylogenetic tree (also phylogeny or evolutionary tree Felsenstein J. (2004). ''Inferring Phylogenies'' Sinauer Associates: Sunderland, MA.) is a branching diagram or a tree showing the evolutionary relationships among various biological spec ...
with the root in the last universal ancestor (or earlier), and with the tips representing the universe of LSm genes existing today.
Homomeric LSm rings in bacteria and archaea
Based on structure, the known LSm proteins divide into a group consisting of the bacterial LSm proteins (Hfq, YlxS and MscS) and a second group of all other LSm proteins, in accordance with the most recently publishedphylogenetic tree
A phylogenetic tree (also phylogeny or evolutionary tree Felsenstein J. (2004). ''Inferring Phylogenies'' Sinauer Associates: Sunderland, MA.) is a branching diagram or a tree showing the evolutionary relationships among various biological spec ...
s. The three archaeal LSm proteins (Sm1, Sm2 and Sm3) also cluster as a group, distinct from the eukaryote LSm proteins. Both the bacterial and archaeal LSm proteins polymerize to homomeric rings, which is the ancestral condition.
Heteromeric LSm rings in eukaryotes
A series of gene duplications of a single eukaryote LSm gene resulted in most (if not all) of the known eukaryote LSm genes. Each of the seven Sm proteins has greater amino acid sequence homology to a corresponding Lsm protein than to the other Sm proteins. This suggests that an ancestral LSm gene duplicated several times, resulting in seven paralogs. These subsequently diverged from each other so that the ancestral homoheptamer LSm ring became a heteroheptamer ring. Based on the known functions of LSm proteins in eukaryotes and archaea, the ancestral function may have been processing of pre- ribosomal RNA, pre-transfer RNA
Transfer RNA (abbreviated tRNA and formerly referred to as sRNA, for soluble RNA) is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes), that serves as the physical link between the mRNA and the amino ac ...
, and pre- RNase P. Then, according to this hypothesis, the seven ancestral eukaryote LSm genes duplicated again to seven pairs of Sm/LSm paralogs; LSm1/SmB, LSm2/SmD1, LSm3/SmD2, LSm4/SmD3, LSm5/SmE, LSm6/SmF and LSm7/SmG. These two group of seven LSm genes (and the corresponding two kinds of LSm rings) evolved to an Sm ring (requiring RNA) and a Lsm ring (which forms without RNA). The LSm1/LSm8 paralog pair also seems to have originated prior to the last common eukaryote ancestor, for a total of at least 15 LSm protein genes. The SmD1/LSm10 paralog pair and the SmD2/LSm11 paralog pair exist only in animals, fungi, and the amoebozoa
Amoebozoa is a major taxonomic group containing about 2,400 described species of amoeboid protists, often possessing blunt, fingerlike, lobose pseudopods and tubular mitochondrial cristae. In traditional and currently no longer supported classi ...
(sometimes identified as the unikont clade) and appears to be absent in the bikont clade ( chromalveolates, excavates, plants and rhizaria). Therefore, these two gene duplications predated this fundamental split in the eukaryote lineage. The SmB/SmN paralog pair is seen only in the placental mammals, which dates this LSm gene duplication.
Biogenesis of snRNPs
Small nuclear ribonucleoproteins (snRNPs) assemble in a tightly orchestrated and regulated process that involves both thecell nucleus
The cell nucleus (pl. nuclei; from Latin or , meaning ''kernel'' or ''seed'') is a membrane-bound organelle found in eukaryotic cells. Eukaryotic cells usually have a single nucleus, but a few cell types, such as mammalian red blood cells, h ...
and cytoplasm.
References
External links
Pfam entry LSM. Pfam is the Sanger Institute database, which is a collection of protein families and domains.
{{DEFAULTSORT:Lsm Protein families Spliceosome