Isotope dating on:
[Wikipedia]
[Google]
[Amazon]
Radiometric dating, radioactive dating or radioisotope dating is a technique which is used to
 All ordinary
All ordinary
 The basic equation of radiometric dating requires that neither the parent nuclide nor the daughter product can enter or leave the material after its formation. The possible confounding effects of contamination of parent and daughter isotopes have to be considered, as do the effects of any loss or gain of such isotopes since the sample was created. It is therefore essential to have as much information as possible about the material being dated and to check for possible signs of alteration. Precision is enhanced if measurements are taken on multiple samples from different locations of the rock body. Alternatively, if several different minerals can be dated from the same sample and are assumed to be formed by the same event and were in equilibrium with the reservoir when they formed, they should form an
The basic equation of radiometric dating requires that neither the parent nuclide nor the daughter product can enter or leave the material after its formation. The possible confounding effects of contamination of parent and daughter isotopes have to be considered, as do the effects of any loss or gain of such isotopes since the sample was created. It is therefore essential to have as much information as possible about the material being dated and to check for possible signs of alteration. Precision is enhanced if measurements are taken on multiple samples from different locations of the rock body. Alternatively, if several different minerals can be dated from the same sample and are assumed to be formed by the same event and were in equilibrium with the reservoir when they formed, they should form an
 Uranium–lead radiometric dating involves using uranium-235 or uranium-238 to date a substance's absolute age. This scheme has been refined to the point that the error margin in dates of rocks can be as low as less than two million years in two-and-a-half billion years. An error margin of 2–5% has been achieved on younger
Uranium–lead radiometric dating involves using uranium-235 or uranium-238 to date a substance's absolute age. This scheme has been refined to the point that the error margin in dates of rocks can be as low as less than two million years in two-and-a-half billion years. An error margin of 2–5% has been achieved on younger
 Radiocarbon dating is also simply called carbon-14 dating. Carbon-14 is a radioactive isotope of carbon, with a half-life of 5,730 years (which is very short compared with the above isotopes), and decays into nitrogen. In other radiometric dating methods, the heavy parent isotopes were produced by
Radiocarbon dating is also simply called carbon-14 dating. Carbon-14 is a radioactive isotope of carbon, with a half-life of 5,730 years (which is very short compared with the above isotopes), and decays into nitrogen. In other radiometric dating methods, the heavy parent isotopes were produced by
 This involves inspection of a polished slice of a material to determine the density of "track" markings left in it by the spontaneous fission of uranium-238 impurities. The uranium content of the sample has to be known, but that can be determined by placing a plastic film over the polished slice of the material, and bombarding it with slow neutrons. This causes induced fission of 235U, as opposed to the spontaneous fission of 238U. The fission tracks produced by this process are recorded in the plastic film. The uranium content of the material can then be calculated from the number of tracks and the
This involves inspection of a polished slice of a material to determine the density of "track" markings left in it by the spontaneous fission of uranium-238 impurities. The uranium content of the sample has to be known, but that can be determined by placing a plastic film over the polished slice of the material, and bombarding it with slow neutrons. This causes induced fission of 235U, as opposed to the spontaneous fission of 238U. The fission tracks produced by this process are recorded in the plastic film. The uranium content of the material can then be calculated from the number of tracks and the
date
Date or dates may refer to:
*Date (fruit), the fruit of the date palm (''Phoenix dactylifera'')
Social activity
*Dating, a form of courtship involving social activity, with the aim of assessing a potential partner
** Group dating
*Play date, a ...
materials such as rocks or carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
, in which trace radioactive impurities were selectively incorporated when they were formed. The method compares the abundance of a naturally occurring radioactive isotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferr ...
within the material to the abundance of its decay
Decay may refer to:
Science and technology
* Bit decay, in computing
* Software decay, in computing
* Distance decay, in geography
* Decay time (fall time), in electronics
Biology
* Decomposition of organic matter
* Tooth decay (dental caries ...
products, which form at a known constant rate of decay. The use of radiometric dating was first published in 1907 by Bertram Boltwood and is now the principal source of information about the absolute age of rocks and other geological features
Geology () is a branch of natural science concerned with Earth and other astronomical objects, the features or rocks of which it is composed, and the processes by which they change over time. Modern geology significantly overlaps all other Ear ...
, including the age of fossilized life forms or the age of Earth itself, and can also be used to date a wide range of natural and man-made materials.
Together with stratigraphic principles, radiometric dating methods are used in geochronology
Geochronology is the science of determining the age of rocks, fossils, and sediments using signatures inherent in the rocks themselves. Absolute geochronology can be accomplished through radioactive isotopes, whereas relative geochronology is ...
to establish the geologic time scale
The geologic time scale, or geological time scale, (GTS) is a representation of time based on the rock record of Earth. It is a system of chronological dating that uses chronostratigraphy (the process of relating strata to time) and geochr ...
. Among the best-known techniques are radiocarbon dating, potassium–argon dating and uranium–lead dating
Uranium–lead dating, abbreviated U–Pb dating, is one of the oldest and most refined of the radiometric dating schemes. It can be used to date rocks that formed and crystallised from about 1 million years to over 4.5 billion years ago with routi ...
. By allowing the establishment of geological timescales, it provides a significant source of information about the ages of fossil
A fossil (from Classical Latin , ) is any preserved remains, impression, or trace of any once-living thing from a past geological age. Examples include bones, shells, exoskeletons, stone imprints of animals or microbes, objects preserved ...
s and the deduced rates of evolution
Evolution is change in the heritable characteristics of biological populations over successive generations. These characteristics are the expressions of genes, which are passed on from parent to offspring during reproduction. Variation ...
ary change. Radiometric dating is also used to date archaeological materials, including ancient artifacts.
Different methods of radiometric dating vary in the timescale over which they are accurate and the materials to which they can be applied.
Fundamentals
Radioactive decay
matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic part ...
is made up of combinations of chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
s, each with its own atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
, indicating the number of protons in the atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron ...
. Additionally, elements may exist in different isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
s, with each isotope of an element differing in the number of neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
s in the nucleus. A particular isotope of a particular element is called a nuclide. Some nuclides are inherently unstable. That is, at some point in time, an atom of such a nuclide will undergo radioactive decay and spontaneously transform into a different nuclide. This transformation may be accomplished in a number of different ways, including alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
(emission of alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be pr ...
s) and beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
(electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
emission, positron emission, or electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. Thi ...
). Another possibility is spontaneous fission into two or more nuclides.
While the moment in time at which a particular nucleus decays is unpredictable, a collection of atoms of a radioactive nuclide decays exponentially
Exponential may refer to any of several mathematical topics related to exponentiation, including:
*Exponential function, also:
**Matrix exponential, the matrix analogue to the above
*Exponential decay, decrease at a rate proportional to value
*Expo ...
at a rate described by a parameter known as the half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
, usually given in units of years when discussing dating techniques. After one half-life has elapsed, one half of the atoms of the nuclide in question will have decayed into a "daughter" nuclide or decay product. In many cases, the daughter nuclide itself is radioactive, resulting in a decay chain
In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". Most radioisotopes do not decay dire ...
, eventually ending with the formation of a stable (nonradioactive) daughter nuclide; each step in such a chain is characterized by a distinct half-life. In these cases, usually the half-life of interest in radiometric dating is the longest one in the chain, which is the rate-limiting factor in the ultimate transformation of the radioactive nuclide into its stable daughter. Isotopic systems that have been exploited for radiometric dating have half-lives ranging from only about 10 years (e.g., tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of ...
) to over 100 billion years (e.g., samarium-147
Naturally occurring samarium (62Sm) is composed of five stable isotopes, 144Sm, 149Sm, 150Sm, 152Sm and 154Sm, and two extremely long-lived radioisotopes, 147Sm (half life: 1.06 y) and 148Sm (7 y), with 152Sm being the most abundant (26. ...
).
For most radioactive nuclides, the half-life depends solely on nuclear properties and is essentially constant. This is known because decay constants measured by different techniques give consistent values within analytical errors and the ages of the same materials are consistent from one method to another. It is not affected by external factors such as temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measurement, measured with a thermometer.
Thermometers are calibrated in various Conversion of units of temperature, temp ...
, pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
, chemical environment, or presence of a magnetic or electric field. The only exceptions are nuclides that decay by the process of electron capture, such as beryllium-7, strontium-85, and zirconium-89, whose decay rate may be affected by local electron density. For all other nuclides, the proportion of the original nuclide to its decay products changes in a predictable way as the original nuclide decays over time.
This predictability allows the relative abundances of related nuclides to be used as a clock
A clock or a timepiece is a device used to measure and indicate time. The clock is one of the oldest human inventions, meeting the need to measure intervals of time shorter than the natural units such as the day, the lunar month and t ...
to measure the time from the incorporation of the original nuclides into a material to the present. Nature has conveniently provided us with radioactive nuclides that have half-lives which range from considerably longer than the age of the universe
In physical cosmology, the age of the universe is the time elapsed since the Big Bang. Astronomers have derived two different measurements of the age of the universe:
a measurement based on direct observations of an early state of the universe, ...
, to less than a zeptosecond
An order of magnitude of time is usually a decimal prefix or decimal order-of-magnitude quantity together with a base unit of time, like a microsecond or a million years. In some cases, the order of magnitude may be implied (usually 1), like ...
. This allows one to measure a very wide range of ages. Isotopes with very long half-lives are called "stable isotopes," and isotopes with very short half-lives are known as "extinct isotopes."
Decay constant determination
The radioactive decay constant, the probability that an atom will decay per year, is the solid foundation of the common measurement of radioactivity. The accuracy and precision of the determination of an age (and a nuclide's half-life) depends on the accuracy and precision of the decay constant measurement. The in-growth method is one way of measuring the decay constant of a system, which involves accumulating daughter nuclides. Unfortunately for nuclides with high decay constants (which are useful for dating very old samples), long periods of time (decades) are required to accumulate enough decay products in a single sample to accurately measure them. A faster method involves using particle counters to determine alpha, beta or gamma activity, and then dividing that by the number of radioactive nuclides. However, it is challenging and expensive to accurately determine the number of radioactive nuclides. Alternatively, decay constants can be determined by comparing isotope data for rocks of known age. This method requires at least one of the isotope systems to be very precisely calibrated, such as the Pb-Pb system.Accuracy of radiometric dating
 The basic equation of radiometric dating requires that neither the parent nuclide nor the daughter product can enter or leave the material after its formation. The possible confounding effects of contamination of parent and daughter isotopes have to be considered, as do the effects of any loss or gain of such isotopes since the sample was created. It is therefore essential to have as much information as possible about the material being dated and to check for possible signs of alteration. Precision is enhanced if measurements are taken on multiple samples from different locations of the rock body. Alternatively, if several different minerals can be dated from the same sample and are assumed to be formed by the same event and were in equilibrium with the reservoir when they formed, they should form an
The basic equation of radiometric dating requires that neither the parent nuclide nor the daughter product can enter or leave the material after its formation. The possible confounding effects of contamination of parent and daughter isotopes have to be considered, as do the effects of any loss or gain of such isotopes since the sample was created. It is therefore essential to have as much information as possible about the material being dated and to check for possible signs of alteration. Precision is enhanced if measurements are taken on multiple samples from different locations of the rock body. Alternatively, if several different minerals can be dated from the same sample and are assumed to be formed by the same event and were in equilibrium with the reservoir when they formed, they should form an isochron
In the mathematical theory of dynamical systems, an isochron is a set of initial conditions for the system that all lead to the same long-term behaviour.
Mathematical isochron An introductory example
Consider the ordinary differential equation ...
. This can reduce the problem of contamination
Contamination is the presence of a constituent, impurity, or some other undesirable element that spoils, corrupts, infects, makes unfit, or makes inferior a material, physical body, natural environment, workplace, etc.
Types of contamination
...
. In uranium–lead dating
Uranium–lead dating, abbreviated U–Pb dating, is one of the oldest and most refined of the radiometric dating schemes. It can be used to date rocks that formed and crystallised from about 1 million years to over 4.5 billion years ago with routi ...
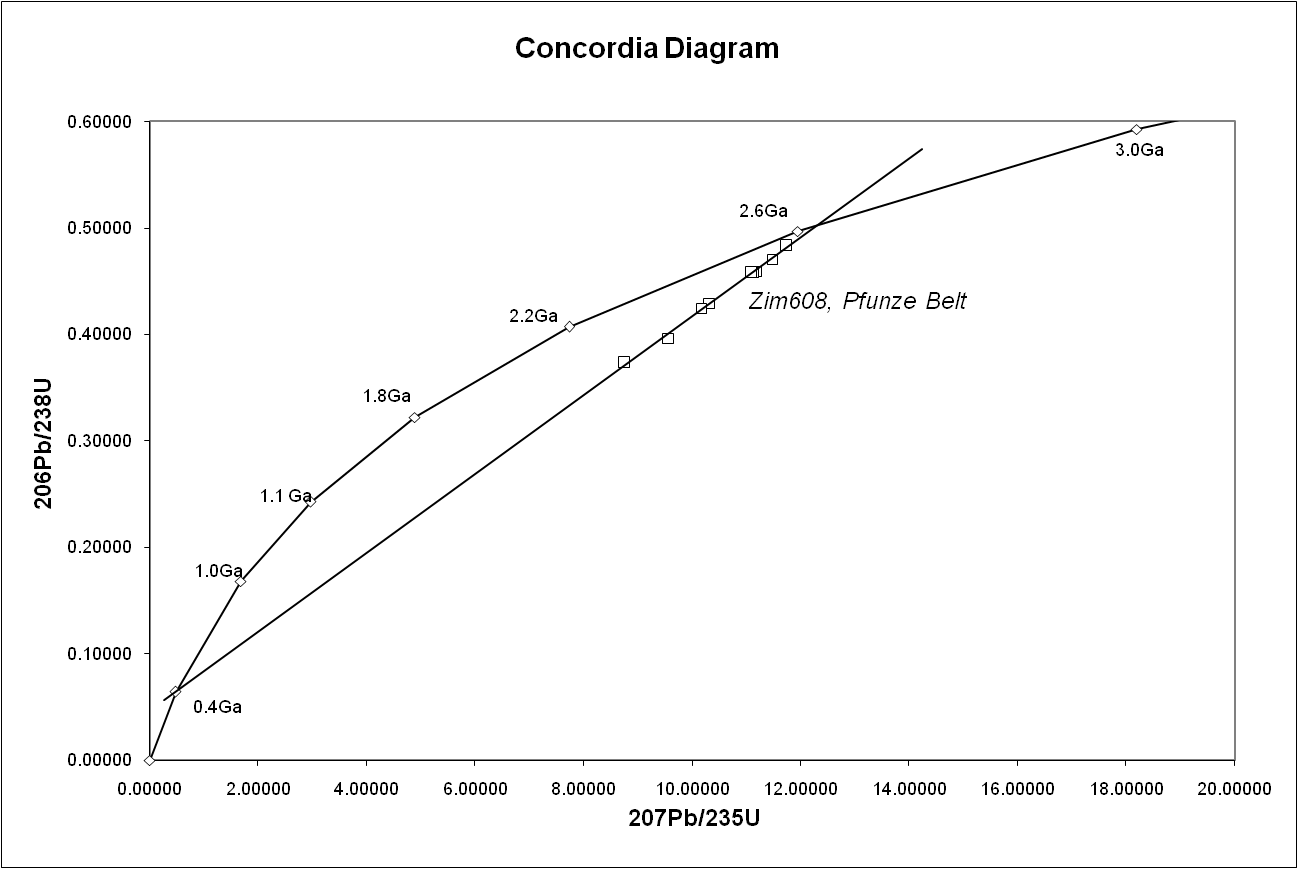
, the concordia diagram is used which also decreases the problem of nuclide loss. Finally, correlation between different isotopic dating methods may be required to confirm the age of a sample. For example, the age of the Amitsoq gneisses from western Greenland was determined to be 3.60 ± 0.05 Ga (billion years ago) using uranium–lead dating and 3.56 ± 0.10 Ga (billion years ago) using lead–lead dating, results that are consistent with each other.
Accurate radiometric dating generally requires that the parent has a long enough half-life that it will be present in significant amounts at the time of measurement (except as described below under "Dating with short-lived extinct radionuclides"), the half-life of the parent is accurately known, and enough of the daughter product is produced to be accurately measured and distinguished from the initial amount of the daughter present in the material. The procedures used to isolate and analyze the parent and daughter nuclides must be precise and accurate. This normally involves isotope-ratio mass spectrometry
Isotope-ratio mass spectrometry (IRMS) is a specialization of mass spectrometry, in which mass spectrometric methods are used to measure the relative abundance of isotopes in a given sample.
This technique has two different applications in the ea ...
.
The precision of a dating method depends in part on the half-life of the radioactive isotope involved. For instance, carbon-14 has a half-life of 5,730 years. After an organism has been dead for 60,000 years, so little carbon-14 is left that accurate dating cannot be established. On the other hand, the concentration of carbon-14 falls off so steeply that the age of relatively young remains can be determined precisely to within a few decades.
Closure temperature
The closure temperature or blocking temperature represents the temperature below which the mineral is a closed system for the studied isotopes. If a material that selectively rejects the daughter nuclide is heated above this temperature, any daughter nuclides that have been accumulated over time will be lost throughdiffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemica ...
, resetting the isotopic "clock" to zero. As the mineral cools, the crystal structure begins to form and diffusion of isotopes is less easy. At a certain temperature, the crystal structure has formed sufficiently to prevent diffusion of isotopes. Thus an igneous or metamorphic rock or melt, which is slowly cooling, does not begin to exhibit measurable radioactive decay until it cools below the closure temperature. The age that can be calculated by radiometric dating is thus the time at which the rock or mineral cooled to closure temperature. This temperature varies for every mineral and isotopic system, so a system can be closed for one mineral but open
Open or OPEN may refer to:
Music
* Open (band), Australian pop/rock band
* The Open (band), English indie rock band
* ''Open'' (Blues Image album), 1969
* ''Open'' (Gotthard album), 1999
* ''Open'' (Cowboy Junkies album), 2001
* ''Open'' ( ...
for another. Dating of different minerals and/or isotope systems (with differing closure temperatures) within the same rock can therefore enable the tracking of the thermal history of the rock in question with time, and thus the history of metamorphic events may become known in detail. These temperatures are experimentally determined in the lab by artificially resetting sample minerals using a high-temperature furnace. This field is known as thermochronology or thermochronometry.
The age equation
The mathematical expression that relates radioactive decay to geologic time is where * is age of the sample, * is number of atoms of the radiogenic daughter isotope in the sample, * is number of atoms of the daughter isotope in the original or initial composition, * is number of atoms of the parent isotope in the sample at time (the present), given by , and * is the decay constant of the parent isotope, equal to the inverse of the radioactivehalf-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of the parent isotope times the natural logarithm of 2.
The equation is most conveniently expressed in terms of the measured quantity ''N''(''t'') rather than the constant initial value ''No''.
To calculate the age, it is assumed that the system is closed (neither parent nor daughter isotopes have been lost from system), ''D''0 must be either negligible or can be accurately estimated, ''λ'' is known to a high precision, and one has accurate and precise measurements of D* and ''N''(''t'').
The above equation makes use of information on the composition of parent and daughter isotopes at the time the material being tested cooled below its closure temperature
In radiometric dating, closure temperature or blocking temperature refers to the temperature of a system, such as a mineral, at the time given by its radiometric date. In physical terms, the closure temperature is the temperature at which a syste ...
. This is well-established for most isotopic systems. However, construction of an isochron does not require information on the original compositions, using merely the present ratios of the parent and daughter isotopes to a standard isotope. An isochron plot is used to solve the age equation graphically and calculate the age of the sample and the original composition.
Modern dating methods
Radiometric dating has been carried out since 1905 when it was invented byErnest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson, (30 August 1871 – 19 October 1937) was a New Zealand physicist who came to be known as the father of nuclear physics.
''Encyclopædia Britannica'' considers him to be the greatest ...
as a method by which one might determine the age of the Earth. In the century since then the techniques have been greatly improved and expanded. Dating can now be performed on samples as small as a nanogram using a mass spectrometer
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is us ...
. The mass spectrometer was invented in the 1940s and began to be used in radiometric dating in the 1950s. It operates by generating a beam of ionized atoms from the sample under test. The ions then travel through a magnetic field, which diverts them into different sampling sensors, known as "Faraday cup
A Faraday cup is a metal (conductive) cup designed to catch charged particles in vacuum. The resulting current can be measured and used to determine the number of ions or electrons hitting the cup. The Faraday cup was named after Michael Fara ...
s", depending on their mass and level of ionization. On impact in the cups, the ions set up a very weak current that can be measured to determine the rate of impacts and the relative concentrations of different atoms in the beams.
Uranium–lead dating method
 Uranium–lead radiometric dating involves using uranium-235 or uranium-238 to date a substance's absolute age. This scheme has been refined to the point that the error margin in dates of rocks can be as low as less than two million years in two-and-a-half billion years. An error margin of 2–5% has been achieved on younger
Uranium–lead radiometric dating involves using uranium-235 or uranium-238 to date a substance's absolute age. This scheme has been refined to the point that the error margin in dates of rocks can be as low as less than two million years in two-and-a-half billion years. An error margin of 2–5% has been achieved on younger Mesozoic
The Mesozoic Era ( ), also called the Age of Reptiles, the Age of Conifers, and colloquially as the Age of the Dinosaurs is the second-to-last era of Earth's geological history, lasting from about , comprising the Triassic, Jurassic and Cretace ...
rocks.
Uranium–lead dating is often performed on the mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2 ...
zircon
Zircon () is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is Zr SiO4. An empirical formula showing some of t ...
(ZrSiO4), though it can be used on other materials, such as baddeleyite and monazite
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the ceriu ...
(see: monazite geochronology
Monazite geochronology is a dating technique to study geological history using the mineral monazite. It is a powerful tool in studying the complex history of metamorphic rocks particularly, as well as igneous, sedimentary and hydrothermal rocks. ...
). Zircon and baddeleyite incorporate uranium atoms into their crystalline structure as substitutes for zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'' ...
, but strongly reject lead. Zircon has a very high closure temperature, is resistant to mechanical weathering and is very chemically inert. Zircon also forms multiple crystal layers during metamorphic events, which each may record an isotopic age of the event. ''In situ'' micro-beam analysis can be achieved via laser ICP-MS
Inductively coupled plasma mass spectrometry (ICP-MS) is a type of mass spectrometry that uses an inductively coupled plasma to ionize the sample. It atomizes the sample and creates atomic and small polyatomic ions, which are then detected. It is ...
or SIMS
Sims, sims or SIMS may refer to:
Games
* ''The Sims'', a life simulation video game series
** ''The Sims'' (video game), the first installment, released in 2000
** ''The Sims 2'', the second installment, released in 2004
** '' The Sims 3'', th ...
techniques.
One of its great advantages is that any sample provides two clocks, one based on uranium-235's decay to lead-207 with a half-life of about 700 million years, and one based on uranium-238's decay to lead-206 with a half-life of about 4.5 billion years, providing a built-in crosscheck that allows accurate determination of the age of the sample even if some of the lead has been lost. This can be seen in the concordia diagram, where the samples plot along an errorchron (straight line) which intersects the concordia curve at the age of the sample.
Samarium–neodymium dating method
This involves thealpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
of 147Sm to 143Nd with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of 1.06 x 1011 years. Accuracy levels of within twenty million years in ages of two-and-a-half billion years are achievable.
Potassium–argon dating method
This involveselectron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. Thi ...
or positron decay of potassium-40 to argon-40. Potassium-40 has a half-life of 1.3 billion years, so this method is applicable to the oldest rocks. Radioactive potassium-40 is common in micas, feldspar
Feldspars are a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagioclase'' (sodium-calcium) felds ...
s, and hornblende
Hornblende is a complex inosilicate series of minerals. It is not a recognized mineral in its own right, but the name is used as a general or field term, to refer to a dark amphibole. Hornblende minerals are common in igneous and metamorphic rock ...
s, though the closure temperature is fairly low in these materials, about 350 °C (mica) to 500 °C (hornblende).
Rubidium–strontium dating method
This is based on the beta decay ofrubidium-87
Rubidium (37Rb) has 36 isotopes, with naturally occurring rubidium being composed of just two isotopes; 85Rb (72.2%) and the radioactive 87Rb (27.8%). Normal mixes of rubidium are radioactive enough to fog photographic film in approximately 30 to ...
to strontium-87
The alkaline earth metal strontium (38Sr) has four stable, naturally occurring isotopes: 84Sr (0.56%), 86Sr (9.86%), 87Sr (7.0%) and 88Sr (82.58%). Its standard atomic weight is 87.62(1).
Only 87Sr is radiogenic; it is produced by decay from th ...
, with a half-life of 50 billion years. This scheme is used to date old igneous
Igneous rock (derived from the Latin word ''ignis'' meaning fire), or magmatic rock, is one of the three main rock types, the others being sedimentary and metamorphic. Igneous rock is formed through the cooling and solidification of magma or ...
and metamorphic rock
Metamorphic rocks arise from the transformation of existing rock to new types of rock in a process called metamorphism. The original rock ( protolith) is subjected to temperatures greater than and, often, elevated pressure of or more, caus ...
s, and has also been used to date lunar samples
Lunar most commonly means "of or relating to the Moon".
Lunar may also refer to:
Arts and entertainment
* ''Lunar'' (series), a series of video games
* "Lunar" (song), by David Guetta
* "Lunar", a song by Priestess from the 2009 album ''Prior t ...
. Closure temperatures are so high that they are not a concern. Rubidium-strontium dating is not as precise as the uranium-lead method, with errors of 30 to 50 million years for a 3-billion-year-old sample. Application of in situ analysis (Laser-Ablation ICP-MS) within single mineral grains in faults have shown that the Rb-Sr method can be used to decipher episodes of fault movement.
Uranium–thorium dating method
A relatively short-range dating technique is based on the decay of uranium-234 into thorium-230, a substance with a half-life of about 80,000 years. It is accompanied by a sister process, in which uranium-235 decays into protactinium-231, which has a half-life of 32,760 years. Whileuranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
is water-soluble, thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
and protactinium
Protactinium (formerly protoactinium) is a chemical element with the symbol Pa and atomic number 91. It is a dense, silvery-gray actinide metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds ...
are not, and so they are selectively precipitated into ocean-floor sediment
Sediment is a naturally occurring material that is broken down by processes of weathering and erosion, and is subsequently transported by the action of wind, water, or ice or by the force of gravity acting on the particles. For example, sa ...
s, from which their ratios are measured. The scheme has a range of several hundred thousand years. A related method is ionium–thorium dating, which measures the ratio of ionium
Thorium (90Th) has seven naturally occurring isotopes but none are stable. One isotope, 232Th, is ''relatively'' stable, with a half-life of 1.405×1010 years, considerably longer than the age of the Earth, and even slightly longer than the gene ...
(thorium-230) to thorium-232 in ocean sediment
Marine sediment, or ocean sediment, or seafloor sediment, are deposits of insoluble particles that have accumulated on the seafloor. These particles have their origins in soil and rocks and have been transported from the land to the sea, mai ...
.
Radiocarbon dating method
 Radiocarbon dating is also simply called carbon-14 dating. Carbon-14 is a radioactive isotope of carbon, with a half-life of 5,730 years (which is very short compared with the above isotopes), and decays into nitrogen. In other radiometric dating methods, the heavy parent isotopes were produced by
Radiocarbon dating is also simply called carbon-14 dating. Carbon-14 is a radioactive isotope of carbon, with a half-life of 5,730 years (which is very short compared with the above isotopes), and decays into nitrogen. In other radiometric dating methods, the heavy parent isotopes were produced by nucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in ...
in supernovas, meaning that any parent isotope with a short half-life should be extinct by now. Carbon-14, though, is continuously created through collisions of neutrons generated by cosmic rays
Cosmic rays are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the Solar System in our ow ...
with nitrogen in the upper atmosphere Upper atmosphere is a collective term that refers to various layers of the atmosphere of the Earth above the troposphere and corresponding regions of the atmospheres of other planets, and includes:
* The mesosphere, which on Earth lies between th ...
and thus remains at a near-constant level on Earth. The carbon-14 ends up as a trace component in atmospheric carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
(CO2).
A carbon-based life form acquires carbon during its lifetime. Plants acquire it through photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
, and animals acquire it from consumption of plants and other animals. When an organism dies, it ceases to take in new carbon-14, and the existing isotope decays with a characteristic half-life (5730 years). The proportion of carbon-14 left when the remains of the organism are examined provides an indication of the time elapsed since its death. This makes carbon-14 an ideal dating method to date the age of bones or the remains of an organism. The carbon-14 dating limit lies around 58,000 to 62,000 years.
The rate of creation of carbon-14 appears to be roughly constant, as cross-checks of carbon-14 dating with other dating methods show it gives consistent results. However, local eruptions of volcano
A volcano is a rupture in the Crust (geology), crust of a Planet#Planetary-mass objects, planetary-mass object, such as Earth, that allows hot lava, volcanic ash, and volcanic gas, gases to escape from a magma chamber below the surface.
On Ear ...
es or other events that give off large amounts of carbon dioxide can reduce local concentrations of carbon-14 and give inaccurate dates. The releases of carbon dioxide into the biosphere
The biosphere (from Greek βίος ''bíos'' "life" and σφαῖρα ''sphaira'' "sphere"), also known as the ecosphere (from Greek οἶκος ''oîkos'' "environment" and σφαῖρα), is the worldwide sum of all ecosystems. It can also ...
as a consequence of industrialization have also depressed the proportion of carbon-14 by a few percent; conversely, the amount of carbon-14 was increased by above-ground nuclear bomb tests that were conducted into the early 1960s. Also, an increase in the solar wind
The solar wind is a stream of charged particles released from the upper atmosphere of the Sun, called the corona. This plasma mostly consists of electrons, protons and alpha particles with kinetic energy between . The composition of the sol ...
or the Earth's magnetic field above the current value would depress the amount of carbon-14 created in the atmosphere.
Fission track dating method
 This involves inspection of a polished slice of a material to determine the density of "track" markings left in it by the spontaneous fission of uranium-238 impurities. The uranium content of the sample has to be known, but that can be determined by placing a plastic film over the polished slice of the material, and bombarding it with slow neutrons. This causes induced fission of 235U, as opposed to the spontaneous fission of 238U. The fission tracks produced by this process are recorded in the plastic film. The uranium content of the material can then be calculated from the number of tracks and the
This involves inspection of a polished slice of a material to determine the density of "track" markings left in it by the spontaneous fission of uranium-238 impurities. The uranium content of the sample has to be known, but that can be determined by placing a plastic film over the polished slice of the material, and bombarding it with slow neutrons. This causes induced fission of 235U, as opposed to the spontaneous fission of 238U. The fission tracks produced by this process are recorded in the plastic film. The uranium content of the material can then be calculated from the number of tracks and the neutron flux
The neutron flux, φ, is a scalar quantity used in nuclear physics and nuclear reactor physics. It is the total length travelled by all free neutrons per unit time and volume. Equivalently, it can be defined as the number of neutrons travellin ...
.
This scheme has application over a wide range of geologic dates. For dates up to a few million years micas, tektite
Tektites (from grc, τηκτός , meaning 'molten') are gravel-sized bodies composed of black, green, brown or grey natural glass formed from terrestrial debris ejected during meteorite impacts. The term was coined by Austrian geologist Franz ...
s (glass fragments from volcanic eruptions), and meteorites are best used. Older materials can be dated using zircon
Zircon () is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is Zr SiO4. An empirical formula showing some of t ...
, apatite, titanite
Titanite, or sphene (from the Greek ''sphenos'' (σφηνώ), meaning wedge), is a calcium titanium nesosilicate mineral, Ca Ti Si O5. Trace impurities of iron and aluminium are typically present. Also commonly present are rare earth metals ...
, epidote
Epidote is a calcium aluminium iron sorosilicate mineral.
Description
Well developed crystals of epidote, Ca2Al2(Fe3+;Al)(SiO4)(Si2O7)O(OH), crystallizing in the monoclinic system, are of frequent occurrence: they are commonly prismatic in hab ...
and garnet
Garnets () are a group of silicate minerals that have been used since the Bronze Age as gemstones and abrasives.
All species of garnets possess similar physical properties and crystal forms, but differ in chemical composition. The different s ...
which have a variable amount of uranium content. Because the fission tracks are healed by temperatures over about 200 °C the technique has limitations as well as benefits. The technique has potential applications for detailing the thermal history of a deposit.
Chlorine-36 dating method
Large amounts of otherwise rare 36Cl (half-life ~300ky) were produced by irradiation of seawater during atmospheric detonations ofnuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion reactions ( thermonuclear bomb), producing a nuclear explosion. Both bom ...
s between 1952 and 1958. The residence time of 36Cl in the atmosphere is about 1 week. Thus, as an event marker of 1950s water in soil and ground water, 36Cl is also useful for dating waters less than 50 years before the present. 36Cl has seen use in other areas of the geological sciences, including dating ice and sediments.
Luminescence dating methods
Luminescence dating methods are not radiometric dating methods in that they do not rely on abundances of isotopes to calculate age. Instead, they are a consequence ofbackground radiation
Background radiation is a measure of the level of ionizing radiation present in the environment at a particular location which is not due to deliberate introduction of radiation sources.
Background radiation originates from a variety of source ...
on certain minerals. Over time, ionizing radiation is absorbed by mineral grains in sediments and archaeological materials such as quartz
Quartz is a hard, crystalline mineral composed of silica ( silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical ...
and potassium feldspar Potassium feldspar refers to a number of minerals in the feldspar group, and containing potassium:
*Orthoclase ( endmember formula K Al Si3 O8), an important tectosilicate mineral that forms igneous rock
*Microcline, chemically the same as orthocla ...
. The radiation causes charge to remain within the grains in structurally unstable "electron traps". Exposure to sunlight or heat releases these charges, effectively "bleaching" the sample and resetting the clock to zero. The trapped charge accumulates over time at a rate determined by the amount of background radiation at the location where the sample was buried. Stimulating these mineral grains using either light (optically stimulated luminescence
In physics, optically stimulated luminescence (OSL) is a method for measuring doses from ionizing radiation. It is used in at least two applications:
* Luminescence dating of ancient materials: mainly geological sediments and sometimes fired pott ...
or infrared stimulated luminescence dating) or heat (thermoluminescence dating
Thermoluminescence dating (TL) is the determination, by means of measuring the accumulated radiation dose, of the time elapsed since material containing crystalline minerals was either heated (lava, ceramics) or exposed to sunlight (sediment ...
) causes a luminescence signal to be emitted as the stored unstable electron energy is released, the intensity of which varies depending on the amount of radiation absorbed during burial and specific properties of the mineral.
These methods can be used to date the age of a sediment layer, as layers deposited on top would prevent the grains from being "bleached" and reset by sunlight. Pottery shards can be dated to the last time they experienced significant heat, generally when they were fired in a kiln.
Other methods
Other methods include: * Argon–argon (Ar–Ar) * Iodine–xenon (I–Xe) * Lanthanum–barium (La–Ba) * Lead–lead (Pb–Pb) * Lutetium–hafnium (Lu–Hf) *Hafnium–tungsten dating
Hafnium–tungsten dating is a geochronological radiometric dating method utilizing the radioactive decay system of hafnium-182 to tungsten-182. The half-life of the system is million years. Today hafnium-182 is an extinct radionuclide, but ...
(Hf-W)
* Potassium–calcium (K–Ca)
* Rhenium–osmium (Re–Os)
* Uranium–uranium (U–U)
* Krypton–krypton (Kr–Kr)
*Beryllium
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to form m ...
(10Be–9Be)
Dating with decay products of short-lived extinct radionuclides
Absolute radiometric dating requires a measurable fraction of parent nucleus to remain in the sample rock. For rocks dating back to the beginning of the solar system, this requires extremely long-lived parent isotopes, making measurement of such rocks' exact ages imprecise. To be able to distinguish the relative ages of rocks from such old material, and to get a better time resolution than that available from long-lived isotopes, short-lived isotopes that are no longer present in the rock can be used. At the beginning of the solar system, there were several relatively short-lived radionuclides like 26Al, 60Fe, 53Mn, and 129I present within the solar nebula. These radionuclides—possibly produced by the explosion of a supernova—are extinct today, but their decay products can be detected in very old material, such as that which constitutes meteorites. By measuring the decay products of extinct radionuclides with amass spectrometer
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is us ...
and using isochronplots, it is possible to determine relative ages of different events in the early history of the solar system. Dating methods based on extinct radionuclides can also be calibrated with the U-Pb method to give absolute ages. Thus both the approximate age and a high time resolution can be obtained. Generally a shorter half-life leads to a higher time resolution at the expense of timescale.
The 129I – 129Xe chronometer
beta-decays to with a half-life of 16 million years. The iodine-xenon chronometer is an isochron technique. Samples are exposed to neutrons in a nuclear reactor. This converts the only stable isotope of iodine () into via neutron capture followed by beta decay (of ). After irradiation, samples are heated in a series of steps and the xenonisotopic signature
An isotopic signature (also isotopic fingerprint) is a ratio of non-radiogenic ' stable isotopes', stable radiogenic isotopes, or unstable radioactive isotopes of particular elements in an investigated material. The ratios of isotopes in a sample ...
of the gas evolved in each step is analysed. When a consistent / ratio is observed across several consecutive temperature steps, it can be interpreted as corresponding to a time at which the sample stopped losing xenon.
Samples of a meteorite called Shallowater are usually included in the irradiation to monitor the conversion efficiency from to . The difference between the measured / ratios of the sample and Shallowater then corresponds to the different ratios of / when they each stopped losing xenon. This in turn corresponds to a difference in age of closure in the early solar system.
The 26Al – 26Mg chronometer
Another example of short-lived extinct radionuclide dating is the – chronometer, which can be used to estimate the relative ages ofchondrule
A chondrule (from Ancient Greek χόνδρος ''chondros'', grain) is a round grain found in a chondrite. Chondrules form as molten or partially molten droplets in space before being accreted to their parent asteroids. Because chondrites repr ...
s. decays to with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of 720 000 years. The dating is simply a question of finding the deviation from the natural abundance
In physics, natural abundance (NA) refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass (a weighted average, weighted by mole-fraction abundance figures) of these isotopes is the atomi ...
of (the product of decay) in comparison with the ratio of the stable isotopes /.
The excess of (often designated *) is found by comparing the / ratio to that of other Solar System materials.
The – chronometer gives an estimate of the time period for formation of primitive meteorites of only a few million years (1.4 million years for Chondrule formation).
A terminology issue
In a July 2022 paper in the journal ''Applied Geochemistry
''Applied Geochemistry'' is an international peer-reviewed academic journal published by the International Association of GeoChemistry covering research on geochemistry and urban geochemistry that was established in 1986. It is published by Elsev ...
'', the authors proposed that the terms “parent isotope" and "daughter isotope” be avoided in favor of the more descriptive "precursor isotope" and "product isotope", analogous to “precursor ion” and “product ion” in mass spectrometry.
See also
* Hadean zircon *Isotope geochemistry
Isotope geochemistry is an aspect of geology based upon the study of natural variations in the relative abundances of isotopes of various elements. Variations in isotopic abundance are measured by isotope ratio mass spectrometry, and can reveal ...
*Paleopedological record
{{inline citations, date=June 2013
The paleopedological record is, essentially, the fossil record of soils. The paleopedological record consists chiefly of paleosols buried by flood sediments, or preserved at geological unconformities, especially p ...
* Radioactivity
* Radiohalo
*Sensitive high-resolution ion microprobe
The sensitive high-resolution ion microprobe (also sensitive high mass-resolution ion microprobe or SHRIMP) is a large-diameter, double-focusing secondary ion mass spectrometer (SIMS) sector instrument produced by Australian Scientific Instrumen ...
(SHRIMP)
References
Further reading
* * * * * {{Authority control Conservation and restoration of cultural heritage