|
Iodine–xenon Dating
Iodine-129 (129I) is a long-lived radioisotope of iodine which occurs naturally, but also is of special interest in the monitoring and effects of man-made nuclear fission products, where it serves as both tracer and potential radiological contaminant. Formation and decay 129I is one of seven long-lived fission products. It is primarily formed from the Nuclear fission, fission of uranium and plutonium in nuclear reactors. Significant amounts were released into the atmosphere as a result of nuclear weapons testing in the 1950s and 1960s. It is also naturally produced in small quantities, due to the spontaneous fission of natural uranium, by cosmic ray spallation of trace levels of xenon in the atmosphere, and by cosmic ray muons striking tellurium-130. 129I decays with a half-life of 15.7 million years, with low-energy beta particle, beta and gamma ray, gamma emissions, to stable xenon-129 (129Xe). Fission product 129I is one of the seven long-lived fission products that are prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace Radioisotope
A trace radioisotope is a radioisotope that occurs naturally in trace amounts (i.e. extremely small). Generally speaking, trace radioisotopes have half-lives that are short in comparison with the age of the Earth, since primordial nuclides tend to occur in larger than trace amounts. Trace radioisotopes are therefore present only because they are continually produced on Earth by natural processes. Natural processes which produce trace radioisotopes include cosmic ray bombardment of stable nuclides, ordinary alpha and beta decay of the long-lived heavy nuclides, thorium-232, uranium-238, and uranium-235, spontaneous fission of uranium-238, and nuclear transmutation reactions induced by natural radioactivity, such as the production of plutonium-239 and uranium-236 from neutron capture by natural uranium. Elements The elements that occur on Earth only in traces are listed below. Isotopes of other elements (not exhaustive): *Tritium * Beryllium-7 *Beryllium-10 *Carbon-14 *Fluorin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Muon
A muon ( ; from the Greek letter mu (μ) used to represent it) is an elementary particle similar to the electron, with an electric charge of −1 '' e'' and a spin of , but with a much greater mass. It is classified as a lepton. As with other leptons, the muon is not thought to be composed of any simpler particles; that is, it is a fundamental particle. The muon is an unstable subatomic particle with a mean lifetime of , much longer than many other subatomic particles. As with the decay of the non-elementary neutron (with a lifetime around 15 minutes), muon decay is slow (by subatomic standards) because the decay is mediated only by the weak interaction (rather than the more powerful strong interaction or electromagnetic interaction), and because the mass difference between the muon and the set of its decay products is small, providing few kinetic degrees of freedom for decay. Muon decay almost always produces at least three particles, which must include an electron o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Transmutation
Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed. A transmutation can be achieved either by nuclear reactions (in which an outside particle reacts with a nucleus) or by radioactive decay, where no outside cause is needed. Natural transmutation by stellar nucleosynthesis in the past created most of the heavier chemical elements in the known existing universe, and continues to take place to this day, creating the vast majority of the most common elements in the universe, including helium, oxygen and carbon. Most stars carry out transmutation through fusion reactions involving hydrogen and helium, while much larger stars are also capable of fusing heavier elements up to iron late in their evolution. Elements heavier than iron, such as gold or lead, are created through elemental transmutations that can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barn (unit)
A barn (symbol: b) is a metric unit of area equal to (100 fm2). Originally used in nuclear physics for expressing the cross sectional area of nuclei and nuclear reactions, today it is also used in all fields of high-energy physics to express the cross sections of any scattering process, and is best understood as a measure of the probability of interaction between small particles. A barn is approximately the cross-sectional area of a uranium nucleus. The barn is also the unit of area used in nuclear quadrupole resonance and nuclear magnetic resonance to quantify the interaction of a nucleus with an electric field gradient. While the barn never was an SI unit, the SI standards body acknowledged it in the 8th SI Brochure (superseded in 2019) due to its use in particle physics. Etymology During Manhattan Project research on the atomic bomb during World War II, American physicists at Purdue University needed a secretive name for a unit with which to quantify the cross-secti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Cross-section
In nuclear physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. The neutron cross section σ can be defined as the area in cm2 for which the number of neutron-nuclei reactions taking place is equal to the product of the number of incident neutrons that would pass through the area and the number of target nuclei. In conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power of a nuclear power plant. The standard unit for measuring the cross section is the barn, which is equal to 10−28 m2 or 10−24 cm2. The larger the neutron cross section, the more likely a neutron will react with the nucleus. An isotope (or nuclide) can be classified according to its neutron cross section and how it reacts to an incident neutron. Nuclides that tend to absorb a neutron and either decay or keep the neutron in its nucleus are neutron absor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Absorption
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, which are repelled electrostatically. Neutron capture plays a significant role in the cosmic nucleosynthesis of heavy elements. In stars it can proceed in two ways: as a rapid process (r-process) or a slow process (s-process). Nuclei of masses greater than 56 cannot be formed by thermonuclear reactions (i.e., by nuclear fusion) but can be formed by neutron capture. Neutron capture on protons yields a line at 2.223 MeV predicted and commonly observed in solar flares. Neutron capture at small neutron flux At small neutron flux, as in a nuclear reactor, a single neutron is captured by a nucleus. For example, when natural gold (197Au) is irradiated by neutrons (n), the isotope 198Au is formed in a highly excited state, and quickly deca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deep Geological Repository
A deep geological repository is a way of storing hazardous or radioactive waste within a stable geologic environment (typically 200–1000 m deep). It entails a combination of waste form, waste package, engineered seals and geology that is suited to provide a high level of long-term isolation and containment without future maintenance. This will prevent any radioactive dangers. A number of mercury, cyanide and arsenic waste repositories are operating worldwide including Canada (Giant Mine) and Germany (potash mines in Herfa-Neurode and Zielitz) and a number of radioactive waste storages are under construction with the Onkalo in Finland being the most advanced. Principles and background Highly toxic waste that cannot be further recycled must be stored in isolation to avoid contamination of air, ground and underground water. Deep geological repository is a type of long-term storage that isolates waste in geological structures that are expected to be stable for millions of years, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spent Nuclear Fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor (usually at a nuclear power plant). It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and depending on its point along the nuclear fuel cycle, it may have considerably different isotopic constituents. The term "fuel" is slightly confusing, as it implies a combustion of some type, which does not occur in a nuclear power plant. Nevertheless, this term is generally accepted. Nature of spent fuel Nanomaterial properties In the oxide fuel, intense temperature gradients exist that cause fission products to migrate. The zirconium tends to move to the centre of the fuel pellet where the temperature is highest, while the lower-boiling fission products move to the edge of the pellet. The pellet is likely to contain many small bubble-like pores that form during use; the fission product xenon migrates to these voids. Some of this xeno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine-131
Iodine-131 (131I, I-131) is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because 131I is a major fission product of uranium and plutonium, comprising nearly 3% of the total products of fission (by weight). See fission product yield for a comparison with other radioactive fission products. 131I is also a major fission product of uranium-233, produced from thorium. Due to its mode of be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
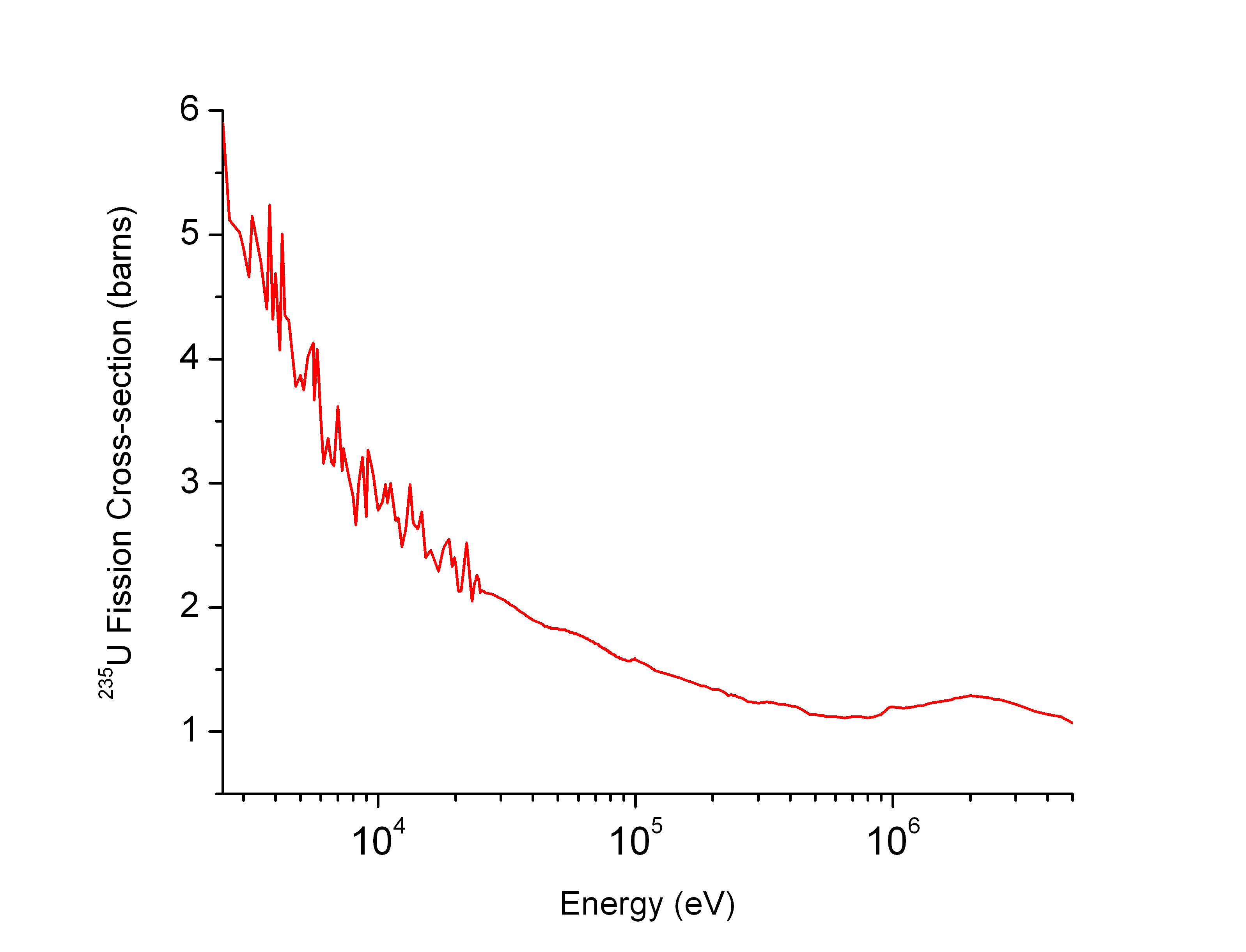
Uranium-235
Uranium-235 (235U or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nature as a primordial nuclide. Uranium-235 has a half-life of 703.8 million years. It was discovered in 1935 by Arthur Jeffrey Dempster. Its fission cross section for slow thermal neutrons is about 584.3±1 barns. For fast neutrons it is on the order of 1 barn. Most but not all neutron absorptions result in fission; a minority result in neutron capture forming uranium-236. Natural decay chain :\begin \ce \begin \ce \\ \ce \end \ce \\ \ce \begin \ce \\ \ce \end \ce \end Fission properties The fission of one atom of uranium-235 releases () inside the reactor. That corresponds to 19.54 TJ/ mol, or 83.14 TJ/kg. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenon-129
Naturally occurring xenon (54Xe) consists of seven stable isotopes and two very long-lived isotopes. Double electron capture has been observed in 124Xe (half-life ) and double beta decay in 136Xe (half-life ), which are among the longest measured half-lives of all nuclides. The isotopes 126Xe and 134Xe are also predicted to undergo double beta decay, but this has never been observed in these isotopes, so they are considered to be stable. Beyond these stable forms, 32 artificial unstable isotopes and various isomers have been studied, the longest-lived of which is 127Xe with a half-life of 36.345 days. All other isotopes have half-lives less than 12 days, most less than 20 hours. The shortest-lived isotope, 108Xe, has a half-life of 58 μs, and is the heaviest known nuclide with equal numbers of protons and neutrons. Of known isomers, the longest-lived is 131mXe with a half-life of 11.934 days. 129Xe is produced by beta decay of 129I (half-life: 16 million years); 131mXe, 133X ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma Ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (), it imparts the highest photon energy. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation ''gamma rays'' based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power. Gamma rays from radioactive decay are in the energy range from a few kiloelectronvolts (keV) to approximately 8 megaelectronvolts (MeV), corresponding to the typical energy levels in nuclei with reasonably long lif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |