|
List Of Radioactive Nuclides By Half-life
This is a list of radioactive nuclides (sometimes also called isotopes), ordered by half-life from shortest to longest, in seconds, minutes, hours, days, and years. Current methods make it difficult to measure half-lives between approximately 10−19 and 10−10 seconds. 10−24 seconds (yoctoseconds) 23 yoctoseconds is the time needed to traverse a 7 femtometre distance at the speed of light, around the diameter of a large atomic nucleus. 10−21 seconds (zeptoseconds) 10−18 seconds (attoseconds) 10−15 seconds (femtoseconds) 10−12 seconds (picoseconds) 10−9 seconds (nanoseconds) 10−6 seconds (microseconds) 10−3 seconds (milliseconds) 100 seconds 103 seconds (kiloseconds) 106 seconds (megaseconds) 109 seconds (gigaseconds) 1012 seconds (teraseconds) 1015 seconds (petaseconds) 1018 seconds (exaseconds) 1021 seconds (zettaseconds) 1024 seconds (yottaseconds) 1027 seconds (ronnaseconds) 1030 seconds (quettaseconds) The half-life of tel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (''t''1/2) for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen-7
Hydrogen (1H) has three naturally occurring Isotope, isotopes, sometimes denoted , , and . and are stable, while has a half-life of years. Heavier isotopes also exist, all of which are synthetic and have a half-life of less than one Orders of magnitude (time)#Zeptosecond, zeptosecond (10−21 s). Of these, is the least stable, while is the most. Hydrogen is the only chemical element, element whose isotopes have different names that remain in common use today: the (or hydrogen-2) isotope is deuterium and the (or hydrogen-3) isotope is tritium. The symbols D and T are sometimes used for deuterium and tritium. The International Union of Pure and Applied Chemistry, IUPAC accepts the D and T symbols, but recommends using standard isotopic symbols ( and ) instead to avoid confusion in the alphabetic sorting of Chemical formula, chemical formulas. The isotope , with no Neutron, neutrons, is sometimes called Hydrogen atom, protium. (During the early study of radioactivity, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium-10
Naturally occurring lithium (3Li) is composed of two stable isotopes, lithium-6 and lithium-7, with the latter being far more abundant on Earth. Both of the natural isotopes have an unexpectedly low nuclear binding energy per nucleon ( for lithium-6 and for lithium-7) when compared with the adjacent lighter and heavier elements, helium ( for helium-4) and beryllium ( for beryllium-9). The longest-lived radioisotope of lithium is lithium-8, which has a half-life of just . Lithium-9 has a half-life of , and lithium-11 has a half-life of . All of the remaining isotopes of lithium have half-lives that are shorter than 10 nanoseconds. The shortest-lived known isotope of lithium is lithium-4, which decays by proton emission with a half-life of about (), although the half-life of lithium-3 is yet to be determined, and is likely to be much shorter, like helium-2 (diproton) which undergoes proton emission within s. Lithium-7 and lithium-6 are two of the primordial nuclides that were ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium-10
Although there are nine known isotopes of helium (2He) (standard atomic weight: ), only helium-3 () and helium-4 () are stable. All radioisotopes are short-lived, the longest-lived being with a half-life of . The least stable is , with a half-life of (), although it is possible that may have an even shorter half-life. In the Earth's atmosphere, the ratio of to is . However, the isotopic abundance of helium varies greatly depending on its origin. In the Local Interstellar Cloud, the proportion of to is , which is times higher than that of atmospheric helium. Rocks from the Earth's crust have isotope ratios varying by as much as a factor of ten; this is used in geology to investigate the origin of rocks and the composition of the Earth's mantle. The different formation processes of the two stable isotopes of helium produce the differing isotope abundances. Equal mixtures of liquid and below separate into two immiscible phases due to differences in quantum statistics: a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Lithium
Naturally occurring lithium (3Li) is composed of two stable isotopes, lithium-6 and lithium-7, with the latter being far more abundant on Earth. Both of the natural isotopes have an unexpectedly low nuclear binding energy per nucleon ( for lithium-6 and for lithium-7) when compared with the adjacent lighter and heavier elements, helium ( for helium-4) and beryllium ( for beryllium-9). The longest-lived radioisotope of lithium is lithium-8, which has a half-life of just . Lithium-9 has a half-life of , and lithium-11 has a half-life of . All of the remaining isotopes of lithium have half-lives that are shorter than 10 nanoseconds. The shortest-lived known isotope of lithium is lithium-4, which decays by proton emission with a half-life of about (), although the half-life of lithium-3 is yet to be determined, and is likely to be much shorter, like helium-2 (diproton) which undergoes proton emission within s. Lithium-7 and lithium-6 are two of the primordial nuclides that wer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium-18
There are 22 isotopes of sodium (11Na), ranging from to , and two isomers ( and ). is the only stable (and the only primordial) isotope. It is considered a monoisotopic element and it has a standard atomic weight of . Sodium has two radioactive cosmogenic isotopes (, with a half-life of ; and , with a half-life of ). With the exception of those two isotopes, all other isotopes have half-lives under a minute, most under a second. The shortest-lived is , with a half-life of seconds. Acute neutron radiation exposure (e.g., from a nuclear criticality accident) converts some of the stable in human blood plasma to . By measuring the concentration of this isotope, the neutron radiation dosage to the victim can be computed. is a positron-emitting isotope with a remarkably long half-life. It is used to create test-objects and point-sources for positron emission tomography. List of isotopes , - , , style="text-align:right" , 11 , style="text-align:right" , 6 , , , p , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen-12
There are three known stable isotopes of oxygen (8O): , , and . Radioactive isotopes ranging from to have also been characterized, all short-lived. The longest-lived radioisotope is with a half-life of , while the shortest-lived isotope is with a half-life of (though the half-lives of the neutron-unbound and are still unknown). List of isotopes , - , , style="text-align:right" , 8 , style="text-align:right" , 3 , , [] , proton emission, 2p , , (3/2−) , , , - , , style="text-align:right" , 8 , style="text-align:right" , 4 , , , 2p , , 0+ , , , - , rowspan=2, , rowspan=2 style="text-align:right" , 8 , rowspan=2 style="text-align:right" , 5 , rowspan=2, , rowspan=2, , β+ () , , rowspan=2, (3/2−) , rowspan=2, , rowspan=2, , - , β+p () , , - , , style="text-align:right" , 8 , style="text-align:right" , 6 , , , β+ , , 0+ , , , - , , style="text-align:right" , 8 , style="text-align:right" , 7 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine-15
Fluorine (9F) has 18 known isotopes ranging from to (with the exception of ) and two isomers ( and ). Only fluorine-19 is stable and naturally occurring in more than trace quantities; therefore, fluorine is a monoisotopic and mononuclidic element. The longest-lived radioisotope is ; it has a half-life of . All other fluorine isotopes have half-lives of less than a minute, and most of those less than a second. The least stable known isotope is , whose half-life is , corresponding to a resonance width of . List of isotopes , - , , style="text-align:right" , 9 , style="text-align:right" , 4 , # , , p ?Decay mode shown is energetically allowed, but has not been experimentally observed to occur in this nuclide. , ? , 1/2+# , , , - , , style="text-align:right" , 9 , style="text-align:right" , 5 , , [] , p ?Decay mode shown is energetically allowed, but has not been experimentally observed to occur in this nuclide. , ? , 2− , , , - , , style= ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force. The diameter of the nucleus is in the range of () for hydrogen (the diameter of a single proton) to about for uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 26,634 (uranium atomic radiu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Femtometre
The magnitudes_.html" ;"title="Magnitude_(mathematics).html" ;"title="atom.html" ;"title="helium helium_atom_and_perspective_Magnitude_(mathematics)">magnitudes_">Magnitude_(mathematics).html"_;"title="atom.html"_;"title="helium_atom">helium_atom_and_perspective_Magnitude_(mathematics)">magnitudes_ The_femtometre_(American_spelling_femtometer)_symbol_fm_derived_from_the_helium_atom_and_perspective_Magnitude_(mathematics)">magnitudes_">Magnitude_(mathematics).html"_;"title="atom.html"_;"title="helium_atom">helium_atom_and_perspective_Magnitude_(mathematics)">magnitudes_ The_femtometre_(American_spelling_femtometer)_symbol_fm_derived_from_the_Danish_language">Danish_and_ helium_atom_and_perspective_Magnitude_(mathematics)">magnitudes_">Magnitude_(mathematics).html"_;"title="atom.html"_;"title="helium_atom">helium_atom_and_perspective_Magnitude_(mathematics)">magnitudes_ The_femtometre_(American_spelling_femtometer)_symbol_fm_derived_from_the_Danish_language">Danish_and_Norwegian_la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
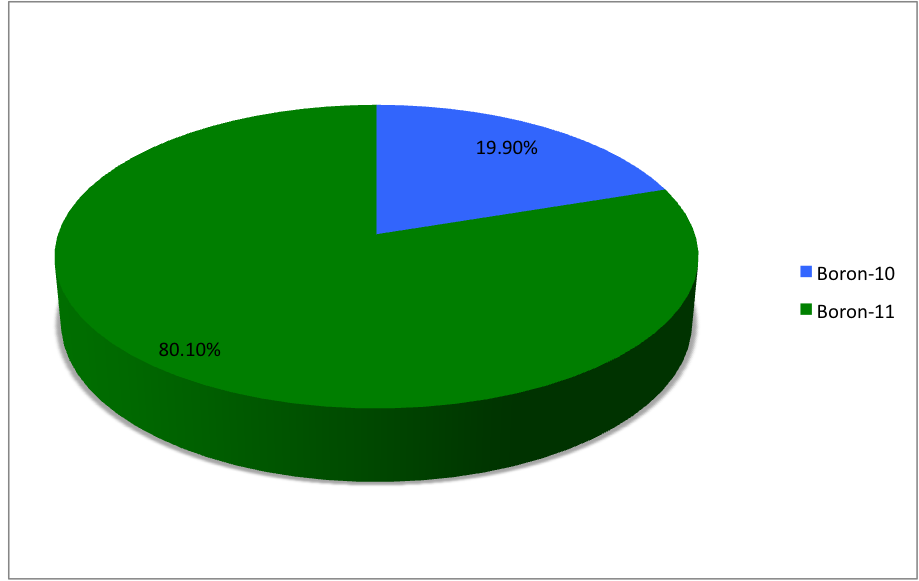
Boron-20
Boron (5B) naturally occurs as isotopes and , the latter of which makes up about 80% of natural boron. There are 13 radioisotopes that have been discovered, with mass numbers from 7 to 21, all with short half-lives, the longest being that of , with a half-life of only and with a half-life of . All other isotopes have half-lives shorter than . Those isotopes with mass below 10 decay into helium (via short-lived isotopes of beryllium for and ) while those with mass above 11 mostly become carbon. List of isotopes , - , ?This isotope has not yet been observed; given data is inferred or estimated from periodic trends. , style="text-align:center" , 5 , style="text-align:center" , 1 , , p-unstable , 2p? , ? , 2−# , , , - , , style="text-align:center" , 5 , style="text-align:center" , 2 , , [] , p , Subsequently decays by double proton emission to for a net reaction of → + 3 , (3/2−) , , , - , Has 1 halo nucleus, halo proton , style="tex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beryllium-15
Beryllium (4Be) has 11 known isotopes and 3 known isomers, but only one of these isotopes () is stable and a primordial nuclide. As such, beryllium is considered a monoisotopic element. It is also a mononuclidic element, because its other isotopes have such short half-lives that none are primordial and their abundance is very low (standard atomic weight is ). Beryllium is unique as being the only monoisotopic element with both an even number of protons and an odd number of neutrons. There are 25 other monoisotopic elements but all have odd atomic numbers, and even numbers of neutrons. Of the 10 radioisotopes of beryllium, the most stable are with a half-life of million years and with a half-life of . All other radioisotopes have half-lives under , most under . The least stable isotope is , with a half-life of . The 1:1 neutron–proton ratio seen in stable isotopes of many light elements (up to oxygen, and in elements with even atomic number up to calcium) is prevented ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |