Ion Chelaru on:
[Wikipedia]
[Google]
[Amazon]
An ion () is an
 Since the electric charge on a proton is equal in magnitude to the charge on an electron, the net electric charge on an ion is equal to the number of protons in the ion minus the number of electrons.
An (−) ( , from the Greek word ἄνω (''ánō''), meaning "up") is an ion with more electrons than protons, giving it a net negative charge (since electrons are negatively charged and protons are positively charged).
A (+) ( , from the Greek word κάτω (''káto''), meaning "down") is an ion with fewer electrons than protons, giving it a positive charge.
There are additional names used for ions with multiple charges. For example, an ion with a −2 charge is known as a
Since the electric charge on a proton is equal in magnitude to the charge on an electron, the net electric charge on an ion is equal to the number of protons in the ion minus the number of electrons.
An (−) ( , from the Greek word ἄνω (''ánō''), meaning "up") is an ion with more electrons than protons, giving it a net negative charge (since electrons are negatively charged and protons are positively charged).
A (+) ( , from the Greek word κάτω (''káto''), meaning "down") is an ion with fewer electrons than protons, giving it a positive charge.
There are additional names used for ions with multiple charges. For example, an ion with a −2 charge is known as a

 The ionizing effect of radiation on a gas is extensively used for the detection of radiation such as
The ionizing effect of radiation on a gas is extensively used for the detection of radiation such as
 When writing the
When writing the  Monatomic ions are sometimes also denoted with Roman numerals, particularly in
Monatomic ions are sometimes also denoted with Roman numerals, particularly in
Na -> Na+ + e-
On the other hand, a Cl + e- -> Cl-
This driving force is what causes sodium and chlorine to undergo a chemical reaction, wherein the "extra" electron is transferred from sodium to chlorine, forming sodium cations and chloride anions. Being oppositely charged, these cations and anions form Na+ + Cl- -> NaCl
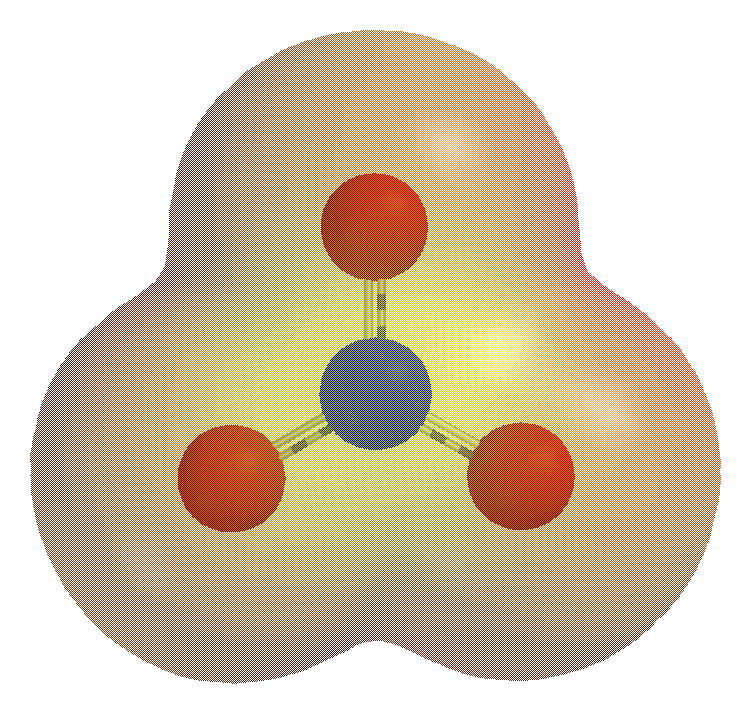 Polyatomic and molecular ions are often formed by the gaining or losing of elemental ions such as a proton, , in neutral molecules. For example, when
Polyatomic and molecular ions are often formed by the gaining or losing of elemental ions such as a proton, , in neutral molecules. For example, when
. Lenntech.com In general, the ionization energy of
atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
or molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
with a net electrical charge
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by ...
.
The charge of an electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
is considered to be negative by convention and this charge is equal and opposite to the charge of a proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons.
A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force
Coulomb's inverse-square law, or simply Coulomb's law, is an experimental law of physics that quantifies the amount of force between two stationary, electrically charged particles. The electric force between charged bodies at rest is conventiona ...
, so cations and anions attract each other and readily form ionic compound
In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged i ...
s.
Ions consisting of only a single atom are termed atomic or monatomic ion
A monatomic ion (also called simple ion) is an ion consisting of exactly one atom. If an ion contains more than one atom, even if these are of the same element, it is called a polyatomic ion. For example, calcium carbonate consists of the monatomic ...
s, while two or more atoms form molecular ions or polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. The term molecule may or may not ...
s. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and a positive ion. Ions are also created by chemical interactions, such as the dissolution
Dissolution may refer to:
Arts and entertainment Books
* ''Dissolution'' (''Forgotten Realms'' novel), a 2002 fantasy novel by Richard Lee Byers
* ''Dissolution'' (Sansom novel), a 2003 historical novel by C. J. Sansom Music
* Dissolution, in mu ...
of a salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
in liquids, or by other means, such as passing a direct current
Direct current (DC) is one-directional flow of electric charge. An electrochemical cell is a prime example of DC power. Direct current may flow through a conductor such as a wire, but can also flow through semiconductors, insulators, or even ...
through a conducting solution, dissolving an anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ...
via ionization
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
.
History of discovery
The word ''ion'' was coined from Greek neuter present participle of ''ienai'' ( el, ἰέναι), meaning "to go". A cation is something that moves down ( el, κάτω pronounced ''kato'', meaning "down") and an anion is something that moves up ( el, ano ἄνω, meaning "up"). They are so called because ions move toward the electrode of opposite charge. This term was introduced (after a suggestion by the Englishpolymath
A polymath ( el, πολυμαθής, , "having learned much"; la, homo universalis, "universal human") is an individual whose knowledge spans a substantial number of subjects, known to draw on complex bodies of knowledge to solve specific pro ...
William Whewell
William Whewell ( ; 24 May 17946 March 1866) was an English polymath, scientist, Anglican priest, philosopher, theologian, and historian of science. He was Master of Trinity College, Cambridge. In his time as a student there, he achieved dist ...
) by English physicist and chemist Michael Faraday
Michael Faraday (; 22 September 1791 – 25 August 1867) was an English scientist who contributed to the study of electromagnetism and electrochemistry. His main discoveries include the principles underlying electromagnetic inducti ...
in 1834 for the then-unknown species that ''goes'' from one electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials de ...
to the other through an aqueous medium. Faraday did not know the nature of these species, but he knew that since metals dissolved into and entered a solution at one electrode and new metal came forth from a solution at the other electrode; that some kind of substance has moved through the solution in a current. This conveys matter from one place to the other. In correspondence with Faraday, Whewell also coined the words ''anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ...
'' and ''cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in whi ...
'', as well as ''anion'' and ''cation'' as ions that are attracted to the respective electrodes.
Svante Arrhenius
Svante August Arrhenius ( , ; 19 February 1859 – 2 October 1927) was a Swedes, Swedish scientist. Originally a physicist, but often referred to as a chemist, Arrhenius was one of the founders of the science of physical chemistry. He received ...
put forth, in his 1884 dissertation, the explanation of the fact that solid crystalline salts dissociate
Dissociation in chemistry is a general process in which molecules (or ionic compounds such as salts, or complexes) separate or split into other things such as atoms, ions, or radicals, usually in a reversible manner. For instance, when an acid ...
into paired charged particles when dissolved, for which he would win the 1903 Nobel Prize in Chemistry. Arrhenius' explanation was that in forming a solution, the salt dissociates into Faraday's ions, he proposed that ions formed even in the absence of an electric current.
Characteristics
Ions in their gas-like state are highly reactive and will rapidly interact with ions of opposite charge to give neutral molecules or ionic salts. Ions are also produced in the liquid or solid state when salts interact with solvents (for example, water) to produce ''solvated ions'', which are more stable, for reasons involving a combination ofenergy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat a ...
and entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
changes as the ions move away from each other to interact with the liquid. These stabilized species are more commonly found in the environment at low temperatures. A common example is the ions present in seawater, which are derived from dissolved salts.
As charged objects, ions are attracted to opposite electric charges (positive to negative, and vice versa) and repelled by like charges. When they move, their trajectories can be deflected by a magnetic field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to ...
.
Electrons, due to their smaller mass and thus larger space-filling properties as matter waves
Matter waves are a central part of the theory of quantum mechanics, being an example of wave–particle duality. All matter exhibits wave-like behavior. For example, a beam of electrons can be diffracted just like a beam of light or a water wave ...
, determine the size of atoms and molecules that possess any electrons at all. Thus, anions (negatively charged ions) are larger than the parent molecule or atom, as the excess electron(s) repel each other and add to the physical size of the ion, because its size is determined by its electron cloud
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any spe ...
. Cations are smaller than the corresponding parent atom or molecule due to the smaller size of the electron cloud. One particular cation (that of hydrogen) contains no electrons, and thus consists of a single proton - ''much smaller'' than the parent hydrogen atom.
Anions and cations
dianion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
and an ion with a +2 charge is known as a dication
A dication is any cation, of general formula X2+, formed by the removal of two electrons from a neutral species.
Diatomic dications corresponding to stable neutral species (e.g. formed by removal of two electrons from H2) often decay quickly into ...
. A zwitterion
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively- and negatively-charged functional groups.
: With amino acids, for example, in solution a chemical equilibrium wil ...
is a neutral molecule with positive and negative charges at different locations within that molecule.
Cations and anions are measured by their ionic radius
Ionic radius, ''r''ion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the cation ...
and they differ in relative size: "Cations are small, most of them less than 10−10 m (10−8 cm) in radius. But most anions are large, as is the most common Earth anion, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
. From this fact it is apparent that most of the space of a crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
is occupied by the anion and that the cations fit into the spaces between them."
The terms ''anion'' and ''cation'' (for ions that respectively travel to the anode and cathode during electrolysis) were introduced by Michael Faraday in 1834 following his consultation with William Whewell
William Whewell ( ; 24 May 17946 March 1866) was an English polymath, scientist, Anglican priest, philosopher, theologian, and historian of science. He was Master of Trinity College, Cambridge. In his time as a student there, he achieved dist ...
.
Natural occurrences
Ions are ubiquitous innature
Nature, in the broadest sense, is the physics, physical world or universe. "Nature" can refer to the phenomenon, phenomena of the physical world, and also to life in general. The study of nature is a large, if not the only, part of science. ...
and are responsible for diverse phenomena from the luminescence of the Sun to the existence of the Earth's ionosphere
The ionosphere () is the ionized part of the upper atmosphere of Earth, from about to above sea level, a region that includes the thermosphere and parts of the mesosphere and exosphere. The ionosphere is ionized by solar radiation. It plays an ...
. Atoms in their ionic state may have a different color from neutral atoms, and thus light absorption by metal ions gives the color of gemstone
A gemstone (also called a fine gem, jewel, precious stone, or semiprecious stone) is a piece of mineral crystal which, in cut and polished form, is used to make jewelry or other adornments. However, certain rocks (such as lapis lazuli, opal, ...
s. In both inorganic and organic chemistry (including biochemistry), the interaction of water and ions is extremely important; an example is energy that drives the breakdown of adenosine triphosphate ( ATP).
Related technology
Ions can be non-chemically prepared using variousion source
An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.
Electron ionization
Electron ...
s, usually involving high voltage
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to m ...
or temperature. These are used in a multitude of devices such as mass spectrometers
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used ...
, optical emission spectrometer
Optics is the branch of physics that studies the behaviour and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behaviour of visible, ultraviole ...
s, particle accelerators
A particle accelerator is a machine that uses electromagnetic fields to propel charged particles to very high speeds and energies, and to contain them in well-defined beams.
Large accelerators are used for fundamental research in particle ...
, ion implanters, and ion engines
An ion thruster, ion drive, or ion engine is a form of electric propulsion used for spacecraft propulsion. It creates thrust by accelerating ions using electricity.
An ion thruster ionizes a neutral gas by extracting some electrons out of ...
.
As reactive charged particles, they are also used in air purification
An air purifier or air cleaner is a device which removes contaminants from the air in a room to improve indoor air quality. These devices are commonly marketed as being beneficial to allergy sufferers and asthmatics, and at reducing or eliminating ...
by disrupting microbes, and in household items such as smoke detector
A smoke detector is a device that senses smoke, typically as an indicator of fire. Smoke detectors are usually housed in plastic enclosures, typically shaped like a disk about in diameter and thick, but shape and size vary. Smoke can be detecte ...
s.
As signalling and metabolism in organisms are controlled by a precise ionic gradient across membranes
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Bi ...
, the disruption of this gradient contributes to cell death. This is a common mechanism exploited by natural and artificial biocides
A biocide is defined in the European legislation as a chemical substance or microorganism intended to destroy, deter, render harmless, or exert a controlling effect on any harmful organism. The US Environmental Protection Agency (EPA) uses a slig ...
, including the ion channels
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of io ...
gramicidin
Gramicidin, also called gramicidin D, is a mix of ionophoric antibiotics, gramicidin A, B and C, which make up about 80%, 5%, and 15% of the mix, respectively. Each has 2 isoforms, so the mix has 6 different types of gramicidin molecules. They c ...
and amphotericin
Amphotericin B is an antifungal medication used for serious fungal infections and leishmaniasis. The fungal infections it is used to treat include mucormycosis, aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, and cryptococcosis. ...
(a fungicide
Fungicides are biocidal chemical compounds or biological organisms used to kill parasitic fungi or their spores. A fungistatic inhibits their growth. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality, ...
).
Inorganic dissolved ions are a component of total dissolved solids
Total dissolved solids (TDS) is a measure of the dissolved combined content of all inorganic and organic substances present in a liquid in molecular, ionized, or micro-granular ( colloidal sol) suspended form. TDS concentrations are often repor ...
, a widely known indicator of water quality
Water quality refers to the chemical, physical, and biological characteristics of water based on the standards of its usage. It is most frequently used by reference to a set of standards against which compliance, generally achieved through tr ...
.
Detection of ionizing radiation

 The ionizing effect of radiation on a gas is extensively used for the detection of radiation such as
The ionizing effect of radiation on a gas is extensively used for the detection of radiation such as alpha
Alpha (uppercase , lowercase ; grc, ἄλφα, ''álpha'', or ell, άλφα, álfa) is the first letter of the Greek alphabet. In the system of Greek numerals, it has a value of one. Alpha is derived from the Phoenician letter aleph , whic ...
, beta
Beta (, ; uppercase , lowercase , or cursive ; grc, βῆτα, bē̂ta or ell, βήτα, víta) is the second letter of the Greek alphabet. In the system of Greek numerals, it has a value of 2. In Modern Greek, it represents the voiced labiod ...
, gamma
Gamma (uppercase , lowercase ; ''gámma'') is the third letter of the Greek alphabet. In the system of Greek numerals it has a value of 3. In Ancient Greek, the letter gamma represented a voiced velar stop . In Modern Greek, this letter re ...
, and X-rays
An X-ray, or, much less commonly, X-radiation, is a penetrating form of high-energy electromagnetic radiation. Most X-rays have a wavelength ranging from 10 Picometre, picometers to 10 Nanometre, nanometers, corresponding to frequency, ...
. The original ionization event in these instruments results in the formation of an "ion pair"; a positive ion and a free electron, by ion impact by the radiation on the gas molecules. The ionization chamber
The ionization chamber is the simplest type of gas-filled radiation detector, and is widely used for the detection and measurement of certain types of ionizing radiation, including X-rays, gamma rays, and beta particles. Conventionally, the term ...
is the simplest of these detectors, and collects all the charges created by ''direct ionization'' within the gas through the application of an electric field.
The Geiger–Müller tube
The Geiger–Müller tube or G–M tube is the sensing element of the Geiger counter instrument used for the detection of ionizing radiation. It is named after Hans Geiger, who invented the principle in 1908, and Walther Müller, who collaborated w ...
and the proportional counter The proportional counter is a type of gaseous ionization detector device used to measure particles of ionizing radiation. The key feature is its ability to measure the energy of incident radiation, by producing a detector output pulse that is ''prop ...
both use a phenomenon known as a Townsend avalanche
The Townsend discharge or Townsend avalanche is a gas ionisation process where free electrons are accelerated by an electric field, collide with gas molecules, and consequently free additional electrons. Those electrons are in turn accelerated an ...
to multiply the effect of the original ionizing event by means of a cascade effect whereby the free electrons are given sufficient energy by the electric field to release further electrons by ion impact.
Chemistry
Denoting the charged state
chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
for an ion, its net charge is written in superscript immediately after the chemical structure for the molecule/atom. The net charge is written with the magnitude ''before'' the sign; that is, a doubly charged cation is indicated as 2+ instead of +2. However, the magnitude of the charge is omitted for singly charged molecules/atoms; for example, the sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
cation is indicated as and ''not'' .
An alternative (and acceptable) way of showing a molecule/atom with multiple charges is by drawing out the signs multiple times, this is often seen with transition metals. Chemists sometimes circle the sign; this is merely ornamental and does not alter the chemical meaning. All three representations of , , and shown in the figure, are thus equivalent.
spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wa ...
; for example, the example seen above is referred to as or . The Roman numeral designates the ''formal oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
'' of an element, whereas the superscripted Indo-Arabic numerals denote the net charge. The two notations are, therefore, exchangeable for monatomic ions, but the Roman numerals ''cannot'' be applied to polyatomic ions. However, it is possible to mix the notations for the individual metal centre with a polyatomic complex, as shown by the uranyl ion example.
Sub-classes
If an ion containsunpaired electron
In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Each atomic orbital of an atom (specified by the three quantum numbers n, l and m) has a capacity to contain ...
s, it is called a ''radical
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
'' ion. Just like uncharged radicals, radical ions are very reactive. Polyatomic ions containing oxygen, such as carbonate and sulfate, are called ''oxyanion An oxyanion, or oxoanion, is an ion with the generic formula (where A represents a chemical element and O represents an oxygen atom). Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determine ...
s''. Molecular ions that contain at least one carbon to hydrogen bond are called ''organic ions''. If the charge in an organic ion is formally centred on a carbon, it is termed a ''carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...
'' (if positively charged) or ''carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3C ...
'' (if negatively charged).
Formation
Formation of monatomic ions
Monatomic ions are formed by the gain or loss of electrons to thevalence shell
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
(the outer-most electron shell) in an atom. The inner shells of an atom are filled with electrons that are tightly bound to the positively charged atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron i ...
, and so do not participate in this kind of chemical interaction. The process of gaining or losing electrons from a neutral atom or molecule is called ''ionization''.
Atoms can be ionized by bombardment with radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or through a material medium. This includes:
* ''electromagnetic radiation'', such as radio waves, microwaves, infrared, visi ...
, but the more usual process of ionization encountered in chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
is the transfer of electrons between atoms or molecules. This transfer is usually driven by the attaining of stable ("closed shell") electronic configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
s. Atoms will gain or lose electrons depending on which action takes the least energy.
For example, a sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
atom, Na, has a single electron in its valence shell, surrounding 2 stable, filled inner shells of 2 and 8 electrons. Since these filled shells are very stable, a sodium atom tends to lose its extra electron and attain this stable configuration, becoming a sodium cation in the process
:chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
atom, Cl, has 7 electrons in its valence shell, which is one short of the stable, filled shell with 8 electrons. Thus, a chlorine atom tends to ''gain'' an extra electron and attain a stable 8-electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
, becoming a chloride anion in the process:
:ionic bond
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds. ...
s and combine to form sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
, NaCl, more commonly known as table salt.
:Formation of polyatomic and molecular ions
 Polyatomic and molecular ions are often formed by the gaining or losing of elemental ions such as a proton, , in neutral molecules. For example, when
Polyatomic and molecular ions are often formed by the gaining or losing of elemental ions such as a proton, , in neutral molecules. For example, when ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
, , accepts a proton, —a process called protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid, ...
—it forms the ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
ion, . Ammonia and ammonium have the same number of electrons in essentially the same electronic configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
, but ammonium has an extra proton that gives it a net positive charge.
Ammonia can also lose an electron to gain a positive charge, forming the ion . However, this ion is unstable, because it has an incomplete valence shell
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
around the nitrogen atom, making it a very reactive radical
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
ion.
Due to the instability of radical ions, polyatomic and molecular ions are usually formed by gaining or losing elemental ions such as , rather than gaining or losing electrons. This allows the molecule to preserve its stable electronic configuration while acquiring an electrical charge.
Ionization potential
Theenergy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat a ...
required to detach an electron in its lowest energy state from an atom or molecule of a gas with less net electric charge is called the ''ionization potential'', or ''ionization energy''. The ''n''th ionization energy of an atom is the energy required to detach its ''n''th electron after the first electrons have already been detached.
Each successive ionization energy is markedly greater than the last. Particularly great increases occur after any given block of atomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any spe ...
s is exhausted of electrons. For this reason, ions tend to form in ways that leave them with full orbital blocks. For example, sodium has one ''valence electron
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
'' in its outermost shell, so in ionized form it is commonly found with one lost electron, as . On the other side of the periodic table, chlorine has seven valence electrons, so in ionized form it is commonly found with one gained electron, as . Caesium has the lowest measured ionization energy of all the elements and helium has the greatest.Chemical elements listed by ionization energy. Lenntech.com In general, the ionization energy of
metals
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
is much lower than the ionization energy of nonmetals
In chemistry, a nonmetal is a chemical element that generally lacks a predominance of metallic properties; they range from colorless gases (like hydrogen) to shiny solids (like carbon, as graphite). The electrons in nonmetals behave differentl ...
, which is why, in general, metals will lose electrons to form positively charged ions and nonmetals will gain electrons to form negatively charged ions.
Ionic bonding
''Ionic bonding'' is a kind ofchemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
ing that arises from the mutual attraction of oppositely charged ions. Ions of like charge repel each other, and ions of opposite charge attract each other. Therefore, ions do not usually exist on their own, but will bind with ions of opposite charge to form a crystal lattice
In geometry and crystallography, a Bravais lattice, named after , is an infinite array of discrete points generated by a set of discrete translation operations described in three dimensional space by
: \mathbf = n_1 \mathbf_1 + n_2 \mathbf_2 + n ...
. The resulting compound is called an ''ionic compound'', and is said to be held together by ''ionic bonding''. In ionic compounds there arise characteristic distances between ion neighbours from which the spatial extension and the ionic radius
Ionic radius, ''r''ion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the cation ...
of individual ions may be derived.
The most common type of ionic bonding is seen in compounds of metals and nonmetals (except noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemi ...
es, which rarely form chemical compounds). Metals are characterized by having a small number of electrons in excess of a stable, closed-shell electronic configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
. As such, they have the tendency to lose these extra electrons in order to attain a stable configuration. This property is known as ''electropositivity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
''. Non-metals, on the other hand, are characterized by having an electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
just a few electrons short of a stable configuration. As such, they have the tendency to gain more electrons in order to achieve a stable configuration. This tendency is known as ''electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
''. When a highly electropositive metal is combined with a highly electronegative nonmetal, the extra electrons from the metal atoms are transferred to the electron-deficient nonmetal atoms. This reaction produces metal cations and nonmetal anions, which are attracted to each other to form a ''salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
''.
Common ions
See also
*Air ionizer
An air ioniser (or negative ion generator or Chizhevsky's chandelier) is a device that uses high voltage to ionise (electrically charge) air molecules. Negative ions, or anions, are particles with one or more extra electrons, conferring a net ne ...
* Aurora
An aurora (plural: auroras or aurorae), also commonly known as the polar lights, is a natural light display in Earth's sky, predominantly seen in high-latitude regions (around the Arctic and Antarctic). Auroras display dynamic patterns of bri ...
* Electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dis ...
* Gaseous ionization detectors
Gaseous ionization detectors are radiation detection instruments used in particle physics to detect the presence of ionizing particles, and in radiation protection applications to measure ionizing radiation.
They use the ionising effect of radia ...
* Ioliomics
Ioliomics (from a portmanteau of ions and liquids) is the study of ions in liquids (or liquid phases) and stipulated with fundamental differences of ionic interactions. Ioliomics covers a broad research area concerning structure, properties and ...
* Ion beam
An ion beam is a type of charged particle beam consisting of ions. Ion beams have many uses in electronics manufacturing (principally ion implantation) and other industries. A variety of ion beam sources exists, some derived from the mercury ...
* Ion exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
* Ionizing radiation
Ionizing radiation (or ionising radiation), including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel ...
* Stopping power of radiation particles
References
{{Authority control Physical chemistry Charge carriers