Heavy Element on:
[Wikipedia]
[Google]
[Amazon]
upright=1.2, Crystals of osmium,_a_heavy_metal_nearly_twice_as_dense_as_lead.html" ;"title="lead.html" ;"title="osmium, a heavy metal nearly twice as dense as lead">osmium, a heavy metal nearly twice as dense as lead">lead.html" ;"title="osmium, a heavy metal nearly twice as dense as lead">osmium, a heavy metal nearly twice as dense as lead
Heavy metals are generally defined as metals with relatively high density, densities, atomic weights, or
File:Chromium crystals and 1cm3 cube.jpg, alt=A silvery finger of chromium irregularly encrusted with diamond-like chunks of chromium of varying size. There is also a one-third sized version of the finger and three roughly hewn gem-like chunks of chromium, as well as the cube. There is a partial reflection of one of the three gem-like chunks in one of the faces of the cube.,
and 1 cm3 cube
File:Arsen 1a.jpg, alt=Two dull silver clusters of crystalline shards,
container to stop tarnishing
File:Cadmium-crystal bar.jpg, alt=A more or less smooth silvery finger of cadmium with some slightly angled faces plus a dull cube,
and 1 cm3 cube
File:Pouring liquid mercury bionerd.jpg, alt=A silvery molasses- like liquid being poured into a circular container with a height equivalent to a smaller coin on its edge,
poured into a
File:Lead electrolytic and 1cm3 cube.jpg, alt=Three, dark broccoli shaped clumps of oxidised lead with grossly distended buds, and a cube of lead which has a dull silvery appearance.,
nodules and 1 cm3 cube
Lead is the most prevalent heavy metal contaminant. Levels in the aquatic environments of industrialised societies have been estimated to be two to three times those of pre-industrial levels. As a component of
 Some uses of heavy metals, including in sport,
Some uses of heavy metals, including in sport,
 The strength or durability of heavy metals such as chromium, iron, nickel, copper, zinc, molybdenum, tin, tungsten, and lead, as well as their alloys, makes them useful for the manufacture of artefacts such as tools, machinery, domestic appliances, appliances, utensils, pipes, railroad tracks, buildings and bridges, automobiles, locks, furniture, ships, planes, coinage and jewellery. They are also used as alloying additives for enhancing the properties of other metals. Of the two dozen elements that have been used in the world's monetised coinage only two, carbon and aluminium, are not heavy metals. Gold, silver, and platinum are used in jewellery as are (for example) nickel, copper, indium, and cobalt in colored gold, coloured gold. Costume jewelry, Low-cost jewellery and toy#Safety regulations, children's toys may be made, to a significant degree, of heavy metals such as chromium, nickel, cadmium, or lead.
Copper, zinc, tin, and lead are mechanically weaker metals but have useful corrosion prevention properties. While each of them will react with air, the resulting patinas of either various copper salts, zinc carbonate, tin dioxide, tin oxide, or a mixture of lead(II) oxide, lead oxide, lead carbonate, carbonate, and Lead sulfate, sulfate, confer valuable passivation (chemistry), protective properties. Copper and lead are therefore used, for example, as roofing materials; zinc acts as an anti-corrosion agent in galvanised steel; and tin serves a similar purpose on steel cans.
The workability and corrosion resistance of iron and chromium are increased by adding gadolinium; the creep (deformation), creep resistance of nickel is improved with the addition of thorium. Tellurium is added to copper (tellurium copper) and steel alloys to improve their machinability; and to lead to make it harder and more acid-resistant.
The strength or durability of heavy metals such as chromium, iron, nickel, copper, zinc, molybdenum, tin, tungsten, and lead, as well as their alloys, makes them useful for the manufacture of artefacts such as tools, machinery, domestic appliances, appliances, utensils, pipes, railroad tracks, buildings and bridges, automobiles, locks, furniture, ships, planes, coinage and jewellery. They are also used as alloying additives for enhancing the properties of other metals. Of the two dozen elements that have been used in the world's monetised coinage only two, carbon and aluminium, are not heavy metals. Gold, silver, and platinum are used in jewellery as are (for example) nickel, copper, indium, and cobalt in colored gold, coloured gold. Costume jewelry, Low-cost jewellery and toy#Safety regulations, children's toys may be made, to a significant degree, of heavy metals such as chromium, nickel, cadmium, or lead.
Copper, zinc, tin, and lead are mechanically weaker metals but have useful corrosion prevention properties. While each of them will react with air, the resulting patinas of either various copper salts, zinc carbonate, tin dioxide, tin oxide, or a mixture of lead(II) oxide, lead oxide, lead carbonate, carbonate, and Lead sulfate, sulfate, confer valuable passivation (chemistry), protective properties. Copper and lead are therefore used, for example, as roofing materials; zinc acts as an anti-corrosion agent in galvanised steel; and tin serves a similar purpose on steel cans.
The workability and corrosion resistance of iron and chromium are increased by adding gadolinium; the creep (deformation), creep resistance of nickel is improved with the addition of thorium. Tellurium is added to copper (tellurium copper) and steel alloys to improve their machinability; and to lead to make it harder and more acid-resistant.
 The biocide, biocidal effects of oligodynamic effect, some heavy metals have been known since antiquity. Platinum, osmium, copper, ruthenium, and other heavy metals, including arsenic, are used in anti-cancer treatments, or have shown potential. Antimony (anti-protozoal), bismuth (antiulcer agent, anti-ulcer), gold (arthritis#Medications, anti-arthritic), and iron (anti-malarial medication, anti-malarial) are also important in medicine. Copper, zinc, silver, gold, or mercury are used in
The biocide, biocidal effects of oligodynamic effect, some heavy metals have been known since antiquity. Platinum, osmium, copper, ruthenium, and other heavy metals, including arsenic, are used in anti-cancer treatments, or have shown potential. Antimony (anti-protozoal), bismuth (antiulcer agent, anti-ulcer), gold (arthritis#Medications, anti-arthritic), and iron (anti-malarial medication, anti-malarial) are also important in medicine. Copper, zinc, silver, gold, or mercury are used in
 The colours of glass, ceramic glazes, environmental impact of paint, paints, pigment#Metal-based pigments, pigments, and
The colours of glass, ceramic glazes, environmental impact of paint, paints, pigment#Metal-based pigments, pigments, and
 Heavy metals or their compounds can be found in electronic components, electrodes, and electrical wiring, wiring and
Heavy metals or their compounds can be found in electronic components, electrodes, and electrical wiring, wiring and
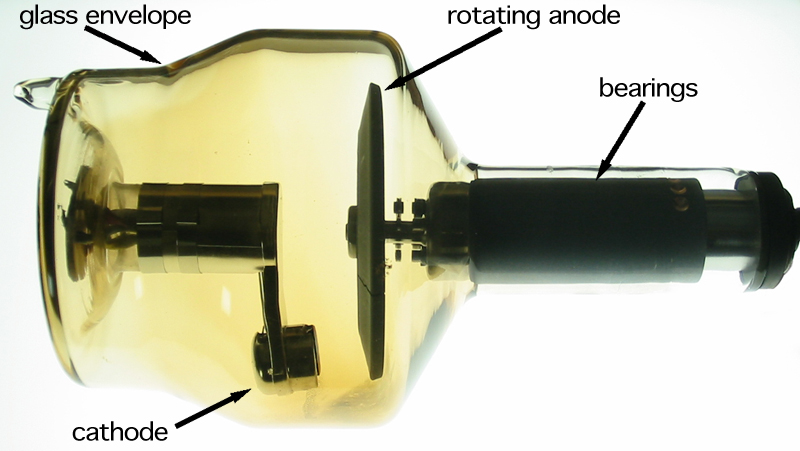 Niche uses of heavy metals with high atomic numbers occur in diagnostic imaging, transmission electron microscopy, electron microscopy, and nuclear science. In diagnostic imaging, heavy metals such as cobalt or tungsten make up the anode materials found in x-ray tubes. In electron microscopy, heavy metals such as lead, gold, palladium, platinum, or uranium are used to make conductive coatings and to introduce electron density into biological specimens by staining, negative staining, or evaporation (deposition), vacuum deposition. In nuclear science, nuclei of heavy metals such as chromium, iron, or zinc are sometimes fired at other heavy metal targets to produce transuranium element#Super-heavy elements, superheavy elements; heavy metals are also employed as spallation#Nuclear spallation, spallation targets for the production of neutrons or radioisotopes such as astatine (using lead, bismuth, thorium, or uranium in the latter case).
Niche uses of heavy metals with high atomic numbers occur in diagnostic imaging, transmission electron microscopy, electron microscopy, and nuclear science. In diagnostic imaging, heavy metals such as cobalt or tungsten make up the anode materials found in x-ray tubes. In electron microscopy, heavy metals such as lead, gold, palladium, platinum, or uranium are used to make conductive coatings and to introduce electron density into biological specimens by staining, negative staining, or evaporation (deposition), vacuum deposition. In nuclear science, nuclei of heavy metals such as chromium, iron, or zinc are sometimes fired at other heavy metal targets to produce transuranium element#Super-heavy elements, superheavy elements; heavy metals are also employed as spallation#Nuclear spallation, spallation targets for the production of neutrons or radioisotopes such as astatine (using lead, bismuth, thorium, or uranium in the latter case).
National Pollutant Inventory
', Department of the Environment and Energy, accessed 16 August 2016. * Baird C. & Cann M. 2012, ''Environmental Chemistry'', 5th ed., W. H. Freeman and Company, New York, . * Baldwin D. R. & Marshall W. J. 1999, "Heavy metal poisoning and its laboratory investigation", ''Annals of Clinical Biochemistry'', vol. 36, no. 3, pp. 267–300, . * Ball J. L., Moore A. D. & Turner S. 2008, ''Ball and Moore's Essential Physics for Radiographers,'' 4th ed., Blackwell Publishing, Chichester, . * Bánfalvi G. 2011, "Heavy metals, trace elements and their cellular effects", in G. Bánfalvi (ed.), ''Cellular Effects of Heavy Metals'', Axel Springer SE, Springer, Dordrecht, pp. 3–28, . * Baranoff E. 2015, "First-row transition metal complexes for the conversion of light into electricity and electricity into light", in W-Y Wong (ed.), ''Organometallics and Related Molecules for Energy Conversion'', Springer, Heidelberg, pp. 61–90, . * Berea E., Rodriguez-lbelo M. & Navarro J. A. R. 2016, "Platinum Group Metal—Organic frameworks" in S. Kaskel (ed.), ''The Chemistry of Metal-Organic Frameworks: Synthesis, Characterisation, and Applications'', vol. 2, Wiley-VCH Weinheim, pp. 203–230, . * Berger A. J. & Bruning N. 1979, ''Lady Luck's Companion: How to Play ... How to Enjoy ... How to Bet ... How to Win'', Harper & Row, New York, . * Berry L. G. & Mason B. 1959, ''Mineralogy: Concepts, Descriptions, Determinations'', W. H. Freeman and Company, San Francisco. * Biddle H. C. & Bush G. L 1949, ''Chemistry Today'', Rand McNally, Chicago. * Bonchev D. & Kamenska V. 1981, "Predicting the properties of the 113–120 transactinide elements", ''The Journal of Physical Chemistry'', vo. 85, no. 9, pp. 1177–1186, . * Bonetti A., Leone R., Muggia F. & Howell S. B. (eds) 2009, ''Platinum and Other Heavy Metal Compounds in Cancer Chemotherapy: Molecular Mechanisms and Clinical Applications'', Humana Press, New York, . * Booth H. S. 1957, ''Inorganic Syntheses'', vol. 5, McGraw-Hill, New York. * Bradl H. E. 2005, "Sources and origins of heavy metals", in Bradl H. E. (ed.), ''Heavy Metals in the Environment: Origin, Interaction and Remediation'', Elsevier, Amsterdam, . * Brady J. E. & Holum J. R. 1995, ''Chemistry: The Study of Matter and its Changes'', 2nd ed., John Wiley & Sons, New York, . * Brephohl E. & Tim McCreight, McCreight T. (ed) 2001, ''The Theory and Practice of Goldsmithing,'' C. Lewton-Brain trans., Brynmorgen Press, Portland, Maine, . * Brown I. 1987, "Astatine: Its organonuclear chemistry and biomedical applications," in H. J. Emeléus & A. G. Sharpe (eds), ''Advances in Inorganic Chemistry'', vol. 31, Academic Press, Orlando, pp. 43–88, . * Bryson R. M. & Hammond C. 2005, "Generic methodologies for nanotechnology: Characterisation"', in R. Kelsall, I. W. Hamley & M. Geoghegan, ''Nanoscale Science and Technology'', John Wiley & Sons, Chichester, pp. 56–129, . * Burkett B. 2010, ''Sport Mechanics for Coaches'', 3rd ed., Human Kinetics, Champaign, Illinois, . * Casey C. 1993, "Restructuring work: New work and new workers in post-industrial production", in R. P. Coulter & I. F. Goodson (eds), ''Rethinking Vocationalism: Whose Work/life is it?'', Our Schools/Our Selves Education Foundation, Toronto, . * Chakhmouradian A.R., Smith M. P. & Kynicky J. 2015, "From "strategic" tungsten to "green" neodymium: A century of critical metals at a glance", ''Ore Geology Reviews'', vol. 64, January, pp. 455–458, . * Ephraim Chambers, Chambers E. 1743,
Metal
, in ''Cyclopedia: Or an Universal Dictionary of Arts and Sciences (etc.)'', vol. 2, D. Midwinter, London. * Chandler D. E. & Roberson R. W. 2009, ''Bioimaging: Current Concepts in Light & Electron Microscopy'', Jones & Bartlett Publishers, Boston, . * Chawla N. & Chawla K. K. 2013, ''Metal matrix composites'', 2nd ed., Springer Science+Business Media, New York, . * Chen J. & Huang K. 2006, "A new technique for extraction of platinum group metals by pressure cyanidation", ''Hydrometallurgy'', vol. 82, nos. 3–4, pp. 164–171, . * Matthew Choptuik, Choptuik M. W., Lehner L. & Pretorias F. 2015, "Probing strong-field gravity through numerical simulation", in Abhay Ashtekar, A. Ashtekar, Beverly Berger, B. K. Berger, J. Isenberg & M. MacCallum (eds), ''General Relativity and Gravitation: A Centennial Perspective'', Cambridge University Press, Cambridge, . * Brian Clegg (writer), Clegg B 2014,
Osmium tetroxide
, ''Chemistry World'', accessed 2 September 2016. * Close F. 2015, ''Nuclear Physics: A Very Short Introduction'', Oxford University Press, Oxford, . * Clugston M & Flemming R 2000, ''Advanced Chemistry'', Oxford University, Oxford, . * Cole M., Lindeque P., Halsband C. & Galloway T. S. 2011, "Microplastics as contaminants in the marine environment: A review", ''Marine Pollution Bulletin'', vol. 62, no. 12, pp. 2588–2597, . * Cole S. E. & Stuart K. R. 2000, "Nuclear and cortical histology for bright-field microscopy, brightfield microscopy", in D. J. Asai & J. D. Forney (eds), ''Methods in Cell Biology'', vol. 62, Academic Press, San Diego, pp. 313–322, . * Cotton S. A. 1997, ''Chemistry of Precious Metals'', Blackie Academic & Professional, London, . * Cotton S. 2006, ''Lanthanide and Actinide Chemistry'', reprinted with corrections 2007, John Wiley & Sons, Chichester, . * Cox P. A. 1997, ''The elements: Their Origin, Abundance and Distribution'', Oxford University Press, Oxford, . * Crundwell F. K., Moats M. S., Ramachandran V., Robinson T. G. & Davenport W. G. 2011, ''Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals'', Elsevier, Kidlington, Oxford, . * Cui X-Y., Li S-W., Zhang S-J., Fan Y-Y., Ma L. Q. 2015, "Toxic metals in children's toys and jewelry: Coupling bioaccessibility with risk assessment", ''Environmental Pollution (journal), Environmental Pollution'', vol. 200, pp. 77–84, . * Dapena J. & Teves M. A. 1982, "Influence of the diameter of the hammer head on the distance of a hammer throw", ''Research Quarterly for Exercise and Sport'', vol. 53, no. 1, pp. 78–81, . * De Zuane J. 1997, ''Handbook of Drinking Water Quality,'' 2nd ed., John Wiley & Sons, New York, . * United States Department of the Navy, Department of the Navy 2009,
Gulf of Alaska Navy Training Activities: Draft Environmental Impact Statement/Overseas Environmental Impact Statement
', U.S. Government, accessed 21 August 2016. * Deschlag J. O. 2011, "Nuclear fission", in A. Vértes, S. Nagy, Z. Klencsár, R. G. Lovas, F. Rösch (eds), ''Handbook of Nuclear Chemistry'', 2nd ed., Springer Science+Business Media, Dordrecht, pp. 223–280, . * Desoize B. 2004, "Metals and metal compounds in cancer treatment", ''Anticancer Research'', vol. 24, no. 3a, pp. 1529–1544, . * Dev N. 2008, 'Modelling Selenium Fate and Transport in Great Salt Lake Wetlands', PhD dissertation, University of Utah, ProQuest, Ann Arbor, Michigan, . * Vincent Di Maio, Di Maio V. J. M. 2001, ''Forensic Pathology,'' 2nd ed., CRC Press, Boca Raton, . * Vincent Di Maio, Di Maio V. J. M. 2016, ''Gunshot Wounds: Practical Aspects of Firearms, Ballistics, and Forensic Techniques'', 3rd ed., CRC Press, Boca Raton, Florida, .
Duffus J. H.
2002,
'Heavy metals'—A meaningless term?"
''Pure and Applied Chemistry'', vol. 74, no. 5, pp. 793–807, . * Dunn P. 2009
''Unusual metals could forge new cancer drugs''
University of Warwick, accessed 23 March 2016. * Ebbing D. D. & Gammon S. D. 2017, ''General Chemistry'', 11th ed., Cengage Learning, Boston, . * Edelstein N. M., Fuger J., Katz J. L. & Morss L. R. 2010, "Summary and comparison of properties of the actinde and transactinide elements," in L. R. Morss, N. M. Edelstein & J. Fuger (eds), ''The Chemistry of the Actinide and Transactinide Elements'', 4th ed., vol. 1–6, Springer Publishing, Springer, Dordrecht, pp. 1753–1835, . * Eisler R. 1993,
Zinc Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review
', Biological Report 10, United States Department of the Interior, U. S. Department of the Interior, Laurel, Maryland, accessed 2 September 2016. * Elliott S. B. 1946, ''The Alkaline-earth and Heavy-metal Soaps, '' Reinhold Publishing Corporation, New York. * John Emsley, Emsley J. 2011,
Nature's Building Blocks
', new edition, Oxford University Press, Oxford, . * Everts S. 2016,
, ''Chemical & Engineering News'', vol. 94, no. 33, pp. 24–26. * Fournier J. 1976, "Bonding and the electronic structure of the actinide metals," ''Journal of Physics and Chemistry of Solids'', vol 37, no. 2, pp. 235–244, . * Frick J. P. (ed.) 2000, ''Woldman's Engineering Alloys'', 9th ed., ASM International (society), ASM International, Materials Park, Ohio, . * Frommer H. H. & Stabulas-Savage J. J. 2014, ''Radiology for the Dental Professional'', 9th ed., Mosby (imprint), Mosby Inc., St. Louis, Missouri, . *Gidding J. C. 1973, ''Chemistry, Man, and Environmental Change: An Integrated Approach'', Canfield Press, New York, . * Leopold Gmelin, Gmelin L. 1849, ''Hand-book of chemistry'', vol. III, Metals, translated from the German by H. Watts, Cavendish Society, London. * Goldsmith R. H. 1982, "Metalloids", ''Journal of Chemical Education'', vol. 59, no. 6, pp. 526–527, . * Gorbachev V. M., Zamyatnin Y. S. & Lbov A. A. 1980, ''Nuclear Reactions in Heavy Elements: A Data Handbook,'' Pergamon Press, Oxford, . * Gordon Gordh, Gordh G. & Headrick D. 2003, ''A Dictionary of Entomology'', CABI Publishing, Wallingford, . * Greenberg B. R. & Patterson D. 2008, ''Art in Chemistry; Chemistry in Art'', 2nd ed., Teachers Ideas Press, Westport, Connecticut, . * Gribbon J. 2016, ''13.8: The Quest to Find the True Age of the Universe and the Theory of Everything'', Yale University Press, New Haven, . * Gschneidner Jr., K. A. 1975, ''Inorganic compounds'', in C. T. Horowitz (ed.), ''Scandium: Its Occurrence, Chemistry, Physics, Metallurgy, Biology and Technology'', Academic Press, London, pp. 152–251, . * Guandalini G. S., Zhang L., Fornero E., Centeno J. A., Mokashi V. P., Ortiz P. A., Stockelman M. D., Osterburg A. R. & Chapman G. G. 2011, "Tissue distribution of tungsten in mice following oral exposure to sodium tungstate," ''Chemical Research in Toxicology'', vol. 24, no. 4, pp 488–493, . * Guney M. & Zagury G. J. 2012, "Heavy metals in toys and low-cost jewelry: Critical review of U.S. and Canadian legislations and recommendations for testing", ''Environmental Science & Technology'', vol. 48, pp. 1238–1246, . * Habashi F. 2009,
Gmelin and his Handbuch"
''Bulletin for the History of Chemistry'', vol. 34, no. 1, pp. 30–1. * Hadhazy A. 2016,
Galactic 'gold mine' explains the origin of nature's heaviest elements
, ''Science Spotlights'', 10 May 2016, accessed 11 July 2016. * William Kenneth Hartmann, Hartmann W. K. 2005, ''Moons & Planets'', 5th ed., Thomson Brooks/Cole, Belmont, California, . * Harvey P. J., Handley H. K. & Taylor M. P. 2015, "Identification of the sources of metal (lead) contamination in drinking waters in north-eastern Tasmania using lead isotopic compositions," ''Environmental Science and Pollution Research'', vol. 22, no. 16, pp. 12276–12288, . * Hasan S. E. 1996, ''Geology and Hazardous Waste Management'', Prentice Hall, Upper Saddle River, New Jersey, . * Hawkes S. J. 1997, "What is a "heavy metal"?", ''Journal of Chemical Education'', vol. 74, no. 11, p. 1374, . * Haynes W. M. 2015, ''CRC Handbook of Chemistry and Physics'', 96th ed., CRC Press, Boca Raton, Florida, . * Hendrickson D. J. 2916, "Effects of early experience on brain and body", in D. Alicata, N. N. Jacobs, A. Guerrero and M. Piasecki (eds), ''Problem-based Behavioural Science and Psychiatry'' 2nd ed., Springer, Cham, pp. 33–54, . * Hermann A., Roald Hoffmann, Hoffmann R. & Neil Ashcroft, Ashcroft N. W. 2013,
Condensed astatine: Monatomic and metallic
, ''Physical Review Letters'', vol. 111, pp. 11604–1−11604-5, . * Herron N. 2000, "Cadmium compounds," in ''Kirk-Othmer Encyclopedia of Chemical Technology'', vol. 4, John Wiley & Sons, New York, pp. 507–523, . * Hoffman D. C., Lee D. M. & Pershina V. 2011, "Transactinide elements and future elements," in L. R. Morss, N. Edelstein, J. Fuger & J. J. Katz (eds), ''The Chemistry of the Actinide and Transactinide Elements'', 4th ed., vol. 3, Springer, Dordrecht, pp. 1652–1752, . * Hofmann S. 2002, ''On Beyond Uranium: Journey to the End of the Periodic Table'', Taylor & Francis, London, . * Housecroft J. E. 2008, ''Inorganic Chemistry'', Elsevier, Burlington, Massachusetts, . * Howell N., Lavers J., Paterson D., Garrett R. & Banati R. 2012,
Trace metal distribution in feathers from migratory, pelagic birds
', Australian Nuclear Science and Technology Organisation, accessed 3 May 2014. * Hübner R., Astin K. B. & Herbert R. J. H. 2010, " 'Heavy metal'—time to move on from semantics to pragmatics?", ''Journal of Environmental Monitoring'', vol. 12, pp. 1511–1514, . * Ikehata K., Jin Y., Maleky N. & Lin A. 2015, "Heavy metal pollution in water resources in China—Occurrence and public health implications", in S. K. Sharma (ed.), ''Heavy Metals in Water: Presence, Removal and Safety,'' Royal Society of Chemistry, Cambridge, pp. 141–167, . * International Antimony Association 2016,
Antimony compounds
', accessed 2 September 2016. * International Platinum Group Metals Association n.d.,
The Primary Production of Platinum Group Metals (PGMs)
', accessed 4 September 2016. * Ismail A. F., Khulbe K. & Matsuura T. 2015, ''Gas Separation Membranes: Polymeric and Inorganic'', Springer, Cham, Switzerland, . * IUPAC 2016,
IUPAC is naming the four new elements nihonium, moscovium, tennessine, and oganesson
accessed 27 August 2016. * Iyengar G. V. 1998, "Reevaluation of the trace element content in Reference Man", ''Radiation Physics and Chemistry,'' vol. 51, nos 4–6, pp. 545–560, * Jackson J. & Summitt J. 2006, ''The Modern Guide to Golf Clubmaking: The Principles and Techniques of Component Golf Club Assembly and Alteration'', 5th ed., Hireko Trading Company, City of Industry, California, . * Järup L 2003, "Hazards of heavy metal contamination", ''British Medical Bulletin'', vol. 68, no. 1, pp. 167–182, . * Jones C. J. 2001, ''d- and f-Block Chemistry'', Royal Society of Chemistry, Cambridge, . * Kantra S. 2001, "What's new", ''Popular Science'', vol. 254, no. 4, April, p. 10. * Keller C., Wolf W. & Shani J. 2012, "Radionuclides, 2. Radioactive elements and artificial radionuclides", in Fritz Ullmann, F. Ullmann (ed.), ''Ullmann's Encyclopedia of Industrial Chemistry'', vol. 31, Wiley-VCH, Weinheim, pp. 89–117, . * King R. B. 1995, ''Inorganic Chemistry of Main Group Elements'', Wiley-VCH, New York, . * Izaak Kolthoff, Kolthoff I. M. & Elving P. J. FR 1964, ''Treatise on Analytical Chemistry'', part II, vol. 6, Interscience Encyclopedia, New York, . * Korenman I. M. 1959, "Regularities in properties of thallium", ''Journal of General Chemistry of the USSR'', English translation, Consultants Bureau, New York, vol. 29, no. 2, pp. 1366–90, . * Kozin L. F. & Hansen S. C. 2013, ''Mercury Handbook: Chemistry, Applications and Environmental Impact'', RSC Publishing, Cambridge, . * Kumar R., Srivastava P. K., Srivastava S. P. 1994, "Leaching of heavy metals (Cr, Fe, and Ni) from stainless steel utensils in food simulates and food materials", ''Bulletin of Environmental Contamination and Toxicology'', vol. 53, no. 2, , pp. 259–266. * Lach K., Steer B., Gorbunov B., Mička V. & Muir R. B. 2015, "Evaluation of exposure to airborne heavy metals at gun shooting ranges", ''The Annals of Occupational Hygiene'', vol. 59, no. 3, pp. 307–323, . * Landis W., Sofield R. & Yu M-H. 2010, ''Introduction to Environmental Toxicology: Molecular Substructures to Ecological Landscapes'', 4th ed., CRC Press, Boca Raton, Florida, . * Lane T. W., Saito M. A., George G. N., Pickering, I. J., Prince R. C. & Morel F. M. M. 2005, "Biochemistry: A cadmium enzyme from a marine diatom", ''Nature (journal), Nature'', vol. 435, no. 7038, p. 42, . * Lee J. D. 1996, ''Concise Inorganic Chemistry,'' 5th ed., Blackwell Science, Oxford, . * Leeper G. W. 1978, ''Managing the Heavy Metals on the Land'' Marcel Dekker, New York, . * Lemly A. D. 1997, "A teratogenic deformity index for evaluating impacts of selenium on fish populations", ''Ecotoxicology and Environmental Safety'', vol. 37, no. 3, pp. 259–266, . * Lide D. R. (ed.) 2004, ''CRC Handbook of Chemistry and Physics'', 85th ed., CRC Press, Boca Raton, Florida, . * Liens J. 2010, "Heavy metals as pollutants", in B. Warf (ed.), ''Encyclopaedia of Geography'', Sage Publications, Thousand Oaks, California, pp. 1415–1418, . * Lima E., Guerra R., Lara V. & Guzmán A. 2013, "Gold nanoparticles as efficient antimicrobial agents for ''Escherichia coli'' and ''Salmonella typhi'' " ''Chemistry Central'', vol. 7:11, . * Litasov K. D. & Shatskiy A. F. 2016, "Composition of the Earth's core: A review", ''Russian Geology and Geophysics'', vol. 57, no. 1, pp. 22–46, . * Livesey A. 2012, ''Advanced Motorsport Engineering'', Routledge, London, . * Livingston R. A. 1991, "Influence of the Environment on the Patina of the Statue of Liberty", ''Environmental Science & Technology,'' vol. 25, no. 8, pp. 1400–1408, . * Longo F. R. 1974, ''General Chemistry: Interaction of Matter, Energy, and Man'', McGraw-Hill, New York, . * Love M. 1998, ''Phasing Out Lead from Gasoline: Worldwide Experience and Policy Implications,'' World Bank Technical Paper volume 397, The World Bank, Washington DC, . * Lyman W. J. 1995, "Transport and transformation processes", in ''Fundamentals of Aquatic Toxicology'', G. M. Rand (ed.), Taylor & Francis, London, pp. 449–492, . * Macintyre J. E. 1994, ''Dictionary of inorganic compounds'', supplement 2, Dictionary of Inorganic Compounds, vol. 7, Chapman & Hall, London, . * MacKay K. M., MacKay R. A. & Henderson W. 2002, ''Introduction to Modern Inorganic Chemistry'', 6th ed., Nelson Thornes, Cheltenham, . * Magee R. J. 1969, ''Steps to Atomic Power'', Cheshire for La Trobe University, Melbourne. * Magill F. N. I (ed.) 1992, ''Magill's Survey of Science'', Physical Science series, vol. 3, Salem Press, Pasadena, . * Martin M. H. & Coughtrey P. J. 1982, ''Biological Monitoring of Heavy Metal Pollution'', Applied Science Publishers, London, . * Massarani M. 2015,
Brazilian mine disaster releases dangerous metals
" ''Chemistry World'', November 2015, accessed 16 April 2016. * Masters C. 1981, ''Homogenous Transition-metal Catalysis: A Gentle Art'', Chapman and Hall, London, . * Matyi R. J. & Baboian R. 1986, "An X-ray Diffraction Analysis of the Patina of the Statue of Liberty", ''Powder Diffraction,'' vol. 1, no. 4, pp. 299–304, . * McColm I. J. 1994, ''Dictionary of Ceramic Science and Engineering'', 2nd ed., Springer Science+Business Media, New York, . * McCurdy R. M. 1975, ''Qualities and quantities: Preparation for College Chemistry'', Harcourt Brace Jovanovich, New York, . * McLemore V. T. (ed.) 2008, ''Basics of Metal Mining Influenced Water'', vol. 1, Society for Mining, Metallurgy, and Exploration, Littleton, Colorado, . * McQueen K. G. 2009, ''Regolith geochemistry'', in K. M. Scott & C. F. Pain (eds), ''Regolith Science'', CSIRO Publishing, Collingwood, Victoria, . * Joseph William Mellor, Mellor J. W. 1924, ''A comprehensive Treatise on Inorganic and Theoretical Chemistry'', vol. 5, Longmans, Green and Company, London. * Moore J. W. & Ramamoorthy S. 1984, ''Heavy Metals in Natural Waters: Applied Monitoring and Impact Assessment'', Springer Verlag, New York, . * Morris C. G. 1992, ''Academic Press Dictionary of Science and Technology'', Harcourt Brace Jovanovich, San Diego, . * Morstein J. H. 2005, "Fat Man", in E. A. Croddy & Y. Y. Wirtz (eds), ''Weapons of Mass Destruction: An Encyclopedia of Worldwide Policy, Technology, and History'', ABC-CLIO, Santa Barbara, California, . * Moselle B. (ed.) 2005, ''2004 National Home Improvement Estimator'', Craftsman Book Company, Carlsbad, California, . * Naja G. M. & Volesky B. 2009, "Toxicity and sources of Pb, Cd, Hg, Cr, As, and radionuclides", in L. K. Wang, J. P. Chen, Y. Hung & N. K. Shammas, ''Heavy Metals in the Environment'', CRC Press, Boca Raton, Florida, . * Nakbanpote W., Meesungneon O. & Prasad M. N. V. 2016, "Potential of ornamental plants for phytoremediation of heavy metals and income generation", in M. N. V. Prasad (ed.), ''Bioremediation and Bioeconomy'', Elsevier, Amsterdam, pp. 179–218, . * Nathans M. W. 1963, ''Elementary Chemistry'', Prentice Hall, Englewood Cliffs, New Jersey. * National Materials Advisory Board 1971, ''Trends in the Use of Depleted Uranium'', National Academy of Sciences – National Academy of Engineering, Washington DC. * National Materials Advisory Board 1973, ''Trends in Usage of Tungsten'', National Academy of Sciences – National Academy of Engineering, Washington DC. * National Organization for Rare Disorders 2015,
Heavy metal poisoning
', accessed 3 March 2016. * Natural Resources Canada 2015,
Generation of the Earth's magnetic field
, accessed 30 August 2016. * Nieboer E. & Richardson D. 1978, "Lichens and 'heavy metals' ", ''International Lichenology Newsletter'', vol. 11, no. 1, pp. 1–3. * Nieboer E. & Richardson D. H. S. 1980, "The replacement of the nondescript term 'heavy metals' by a biologically and chemically significant classification of metal ions", ''Environmental Pollution (journal), Environmental Pollution Series B, Chemical and Physical'', vol. 1, no. 1, pp. 3–26, . * Nzierżanowski K. & Gawroński S. W. 2012,
Heavy metal concentration in plants growing on the vicinity of railroad tracks: a pilot study
, ''Challenges of Modern Technology'', vol. 3, no. 1, pp. 42–45, , accessed 21 August 2016. * Ohlendorf H. M. 2003, "Ecotoxicology of selenium", in D. J. Hoffman, B. A. Rattner, G. A. Burton & John Cairns (biochemist), J. Cairns, ''Handbook of Ecotoxicology'', 2nd ed., Lewis Publishers, Boca Raton, pp. 466–491, . * Ondreička R., Kortus J. & Ginter E. 1971, "Aluminium, its absorption, distribution, and effects on phosphorus metabolism", in S. C. Skoryna & D. Waldron-Edward (eds), ''Intestinal Absorption of Metal Ions, Trace Elements and Radionuclides'', Pergamon press, Oxford. * Ong K. L., Tan T. H. & Cheung W. L. 1997, "Potassium permanganate poisoning—a rare cause of fatal poisoning", ''Journal of Accident & Emergency Medicine'', vol. 14, no. 1, pp. 43–45, . * ''Oxford English Dictionary'' 1989, 2nd ed., Oxford University Press, Oxford, . * Pacheco-Torgal F., Jalali S. & Fucic A. (eds) 2012, ''Toxicity of building materials'', Woodhead Publishing, Oxford, . * Thanu Padmanabhan, Padmanabhan T. 2001, ''Theoretical Astrophysics'', vol. 2, Stars and Stellar Systems, Cambridge University Press, Cambridge, . * Pan W. & Dai J. 2015, "ADS based on linear accelerators", in W. Chao & W. Chou (eds), ''Reviews of accelerator science and technology'', vol. 8, Accelerator Applications in Energy and Security, World Scientific, Singapore, pp. 55–76, . * Parish R. V. 1977, ''The Metallic Elements'', Longman, New York, . * Perry J. & Vanderklein E. L. ''Water Quality: Management of a Natural Resource,'' Blackwell Science, Cambridge, Massachusetts . * Pickering N. C. 1991, ''The Bowed String: Observations on the Design, Manufacture, Testing and Performance of Strings for Violins, Violas and Cellos'', Amereon, Mattituck, New York. * Podosek F. A. 2011, "Noble gases", in H. D. Holland & Karl Turekian, K. K. Turekian (eds), ''Isotope Geochemistry: From the Treatise on Geochemistry'', Elsevier, Amsterdam, pp. 467–492, . * Podsiki C. 2008,
Heavy metals, their salts, and other compounds
, ''American Institute for Conservation, AIC News,'' November, special insert, pp. 1–4. * Preschel J. July 29, 2005,
Green bullets not so eco-friendly
, ''CBS News'', accessed 18 March 2016. * Preuss P. 17 July 2011,
What keeps the Earth cooking?
" Berkeley Lab, accessed 17 July 2016. * Carlos Prieto (cellist), Prieto C. 2011, ''The Adventures of a Cello: Revised Edition, with a New Epilogue,'' University of Texas Press, Austin, * Raghuram P., Soma Raju I. V. & Sriramulu J. 2010, "Heavy metals testing in active pharmaceutical ingredients: an alternate approach", ''Pharmazie'', vol. 65, no. 1, pp. 15–18, . * Rainbow P. S. 1991, "The biology of heavy metals in the sea", in J. Rose (ed.), ''Water and the Environment'', Gordon and Breach Science Publishers, Philadelphia, pp. 415–432, . * Rand G. M., Wells P. G. & McCarty L. S. 1995, "Introduction to aquatic toxicology", in G. M. Rand (ed.), ''Fundamentals of Aquatic Toxicology: Effects, Environmental Fate and Risk Assessment'', 2nd ed., Taylor & Francis, London, pp. 3–70, . * Rankin W. J. 2011, ''Minerals, Metals and Sustainability: Meeting Future Material Needs'', CSIRO Publishing, Collingwood, Victoria, . * Rasic-Milutinovic Z. & Jovanovic D. 2013, "Toxic metals", in M. Ferrante, G. Oliveri Conti, Z. Rasic-Milutinovic & D. Jovanovic (eds), ''Health Effects of Metals and Related Substances in Drinking Water'', International Water Association, IWA Publishing, London, . * Raymond R. 1984, ''Out of the Fiery Furnace: The Impact of Metals on the History of Mankind'', Macmillan Publishing, Macmillan, South Melbourne, . * Rebhandl W., Milassin A., Brunner L., Steffan I., Benkö T., Hörmann M., Burschen J. 2007, "In vitro study of ingested coins: Leave them or retrieve them?", ''Journal of Paediatric Surgery'', vol. 42, no. 10, pp. 1729–1734, . * Rehder D. 2010, ''Chemistry in Space: From Interstellar Matter to the Origin of Life'', Wiley-VCH, Weinheim, . * Renner H., Schlamp G., Kleinwächter I., Drost E., Lüchow H. M., Tews P., Panster P., Diehl M., Lang J., Kreuzer T., Knödler A., Starz K. A., Dermann K., Rothaut J., Drieselmann R., Peter C. & Schiele R. 2012, "Platinum Group Metals and compounds", in F. Ullmann (ed.), ''Ullmann's Encyclopedia of Industrial Chemistry'', vol. 28, Wiley-VCH, Weinheim, pp. 317–388, . * Reyes J. W. 2007,
Environmental Policy as Social Policy? The Impact of Childhood Lead Exposure on Crime
', National Bureau of Economic Research Working Paper 13097, accessed 16 October 2016. * Ian Ridpath, Ridpath I. (ed.) 2012, ''Oxford Dictionary of Astronomy'', 2nd ed. rev., Oxford University Press, New York, . * Rockhoff H. 2012, ''America's Economic Way of War: War and the US Economy from the Spanish–American War to the Persian Gulf War'', Cambridge University Press, Cambridge, . * Roe J. & Roe M. 1992, "World's coinage uses 24 chemical elements", ''World Coinage News'', vol. 19, no. 4, pp. 24–25; no. 5, pp. 18–19. * Russell A. M. & Lee K. L. 2005, ''Structure–Property Relations in Nonferrous Metals'', John Wiley & Sons, Hoboken, New Jersey, . * Rusyniak D. E., Arroyo A., Acciani J., Froberg B., Kao L. & Furbee B. 2010, "Heavy metal poisoning: Management of intoxication and antidotes", in A. Luch (ed.), ''Molecular, Clinical and Environmental Toxicology'', vol. 2, Birkhäuser Verlag, Basel, pp. 365–396, . * Ryan J. 2012, ''Personal Financial Literacy'', 2nd ed., South-Western, Mason, Ohio, . * Samsonov G. V. (ed.) 1968, ''Handbook of the Physicochemical Properties of the Elements'', IFI-Plenum, New York, . * Sanders R. 2003,
Radioactive potassium may be major heat source in Earth's core
" ''UCBerkelyNews'', 10 December, accessed 17 July 20016. * Schweitzer P. A. 2003, ''Metallic materials: Physical, Mechanical, and Corrosion properties'', Marcel Dekker, New York, . * George K. Schweitzer, Schweitzer G. K. & Pesterfield L. L. 2010, ''The Aqueous Chemistry of the Elements'', Oxford University Press, Oxford, . * Scott R. M. 1989, ''Chemical Hazards in the Workplace'', CRC Press, Boca Raton, Orlando, . * Scoullos M. (ed.), Vonkeman G. H., Thornton I. & Makuch Z. 2001, ''Mercury — Cadmium — Lead Handbook for Sustainable Heavy Metals Policy and Regulation'', Kluwer Academic Publishers, Dordrecht, . * Selinger B. 1978, ''Chemistry in the Market Place'', 2nd ed., Australian National University Press, Canberra, . * Seymour R. J. & O'Farrelly J. 2012, "Platinum Group Metals", ''Kirk-Other Encyclopaedia of Chemical Technology'', John Wiley & Sons, New York, . * Shaw B. P., Sahu S. K. & Mishra R. K. 1999, "Heavy metal induced oxidative damage in terrestrial plants", in M. N. V. Prased (ed.), ''Heavy Metal Stress in Plants: From Biomolecules to Ecosystems'' Springer-Verlag, Berlin, . * Shedd K. B. 2002,
Tungsten"
''Minerals Yearbook'', United States Geological Survey. * Nevil Sidgwick, Sidgwick N. V. 1950, ''The Chemical Elements and their Compounds'', vol. 1, Oxford University Press, London. * Silva R. J. 2010, "Fermium, mendelevium, nobelium, and lawrencium", in L. R. Morss, N. Edelstein & J. Fuger (eds), ''The Chemistry of the Actinide and Transactinide Elements'', vol. 3, 4th ed., Springer, Dordrecht, pp. 1621–1651, . * Spolek G. 2007, "Design and materials in fly fishing", in A. Subic (ed.), ''Materials in Sports Equipment'', Volume 2, Woodhead Publishing, Abington, Cambridge, pp. 225–247, . * Stankovic S. & Stankocic A. R. 2013, "Bioindicators of toxic metals", in E. Lichtfouse, J. Schwarzbauer, D. Robert 2013, ''Green materials for energy, products and depollution'', Springer, Dordrecht, , pp. 151–228. * State Water Control Resources Board 1987, ''Toxic substances monitoring program'', issue 79, part 20 of the Water Quality Monitoring Report, Sacramento, California. * Technical Publications 1953, ''Fire Engineering'', vol. 111, p. 235, . * The Minerals, Metals and Materials Society,
Light Metals Division 2016
', accessed 22 June 2016. * ''The
Toxic effects of metals
, in C. D. Klaassen (ed.), ''Casarett and Doull's Toxicology: the Basic Science of Poisons'', 8th ed., McGraw-Hill Medical, New York, , accessed 9 September 2016 . * Tomasik P. & Ratajewicz Z. 1985, ''Pyridine metal complexes,'' vol. 14, no. 6A, The Chemistry of Heterocyclic Compounds, John Wiley & Sons, New York, . * Topp N. E. 1965, ''The Chemistry of the Rare-earth Elements'', Elsevier Publishing Company, Amsterdam. * Torrice M. 2016,
How lead ended up in Flint's tap water
" ''Chemical & Engineering News'', vol. 94, no. 7, pp. 26–27. * Tretkoff E. 2006,
March 20, 1800: Volta describes the Electric Battery
, ''APS News, This Month in Physics History'', American Physical Society, accessed 26 August 2016. * Uden P. C. 2005, 'Speciation of Selenium,' in R. Cornelis, J. Caruso, H. Crews & K. Heumann (eds), ''Handbook of Elemental Speciation II: Species in the Environment, Food, Medicine and Occupational Health,'' John Wiley & Sons, Chichester, pp. 346–65, . * United States Environmental Protection Agency 1988, ''Ambient Aquatic Life Water Quality Criteria for Antimony (III),'' draft, Office of Research and Development, Environmental Research Laboratories, Washington. * United States Environmental Protection Agency 2014,
Technical Fact Sheet–Tungsten
', accessed 27 March 2016. * United States Government 2014,
Toxic Pollutant List
', Code of Federal Regulations, 40 CFR 401.15., accessed 27 March 2016. * Valkovic V. 1990, "Origin of trace element requirements by living matter", in B. Gruber & J. H. Yopp (eds), ''Symmetries in Science IV: Biological and biophysical systems'', Plenum Press, New York, pp. 213–242, . * VanGelder K. T. 2014, ''Fundamentals of Automotive Technology: Principles and Practice'', Jones & Bartlett Learning, Burlington MA, . * Venner M., Lessening M., Pankani D. & Strecker E. 2004,
Identification of Research Needs Related to Highway Runoff Management
', Transportation Research Board, Washington DC, , accessed 21 August 2016. * Venugopal B. & Luckey T. D. 1978, ''Metal Toxicity in Mammals'', vol. 2, Plenum Press, New York, . * Vernon R. E. 2013, "Which elements are metalloids", ''Journal of Chemical Education'', vol. 90, no. 12, pp. 1703–1707, . * Volesky B. 1990, ''Biosorption of Heavy Metals'', CRC Press, Boca Raton, . * von Gleich A. 2013, "Outlines of a sustainable metals industry", in A. von Gleich, R. U. Ayres & S. Gößling-Reisemann (eds), ''Sustainable Metals Management'', Springer, Dordrecht, pp. 3–40, . * von Zeerleder A. 1949, ''Technology of Light Metals'', Elsevier Publishing Company, New York. * Warth A. H. 1956, ''The Chemistry and Technology of Waxes'', Reinhold Publishing Corporation, New York. * Spencer R. Weart, Weart S. R. 1983, "The discovery of nuclear fission and a nuclear physics paradigm", in W. Shea (ed.), ''Otto Hahn and the Rise of Nuclear Physics'', D. Reidel Publishing Company, Dordrecht, pp. 91–133, . * Weber D. J. & Rutula W. A. 2001, "Use of metals as microbicides in preventing infections in healthcare", in ''Disinfection, Sterilization, and Preservation'', 5th ed., S. S. Block (ed.), Lippincott, Williams & Wilkins, Philadelphia, . * Welter G. 1976, ''Cleaning and Preservation of Coins and Medals'', S. J. Durst, New York, . * White C. 2010, ''Projectile Dynamics in Sport: Principles and Applications'', Routledge, London, . * Wiberg N. 2001, ''Inorganic Chemistry'', Academic Press, San Diego, . * Wijayawardena M. A. A., Megharaj M. & Naidu R. 2016, "Exposure, toxicity, health impacts and bioavailability of heavy metal mixtures", in D. L. Sparks, ''Advances in Agronomy'', vol. 138, pp. 175–234, Academic Press, London, . * Wingerson L. 1986,
America cleans up Liberty
, ''New Scientist,'' 25 December/1 January 1987, pp. 31–35, accessed 1 October 2016. * Wong M. Y., Hedley G. J., Xie G., Kölln L. S, Samuel I. D. W., Pertegaś A., Bolink H. J., Mosman-Colman, E., "Light-emitting electrochemical cells and solution-processed organic light-emitting diodes using small molecule organic thermally activated delayed fluorescence emitters", ''Chemistry of Materials'', vol. 27, no. 19, pp. 6535–6542, . * Wulfsberg G. 1987, ''Principles of Descriptive Inorganic Chemistry'', Cengage Learning, Brooks/Cole Publishing Company, Monterey, California, . * Wulfsberg G. 2000, ''Inorganic Chemistry'', University Science Books, Sausalito, California, . * Yadav J. S., Antony A., Subba Reddy, B. V. 2012, "Bismuth(III) salts as synthetic tools in organic transformations", in T. Ollevier (ed.), ''Bismuth-mediated Organic Reactions'', Topics in Current Chemistry 311, Springer, Heidelberg, . * Yang D. J., Jolly W. L. & O'Keefe A. 1977, "Conversion of hydrous germanium(II) oxide to germynyl sesquioxide, (HGe)2O3", ''Inorganic Chemistry (journal), 'Inorganic Chemistry'', vol. 16, no. 11, pp. 2980–2982, . * Yousif N. 2007, ''Geochemistry of stream sediment from the state of Colorado using NURE data'', ETD Collection for the University of Texas, El Paso
paper AAI3273991
2002,
Heavy metals'—A meaningless term?"
''Pure and Applied Chemistry'', vol. 74, no. 5, pp. 793–807, . Includes a survey of the term's various meanings. * Hawkes S. J. 1997,
What is a 'heavy metal'?
, ''Journal of Chemical Education'', vol. 74, no. 11, p. 1374, . A chemist's perspective. * Hübner R., Astin K. B. & Herbert R. J. H. 2010, Heavy metal'—time to move on from semantics to pragmatics?", ''Journal of Environmental Monitoring'', vol. 12, pp. 1511–1514, . Finds that, despite its lack of specificity, the term appears to have become part of the language of science. Toxicity and biological role * Baird C. & Cann M. 2012, ''Environmental Chemistry'', 5th ed., chapter 12, "Toxic heavy metals", W. H. Freeman and Company, New York, . Discusses the use, toxicity, and distribution of Hg, Pb, Cd, As, and Cr. * Nieboer E. & Richardson D. H. S. 1980, "The replacement of the nondescript term 'heavy metals' by a biologically and chemically significant classification of metal ions", ''Environmental Pollution Series B, Chemical and Physical'', vol. 1, no. 1, pp. 3–26, . A widely cited paper, focusing on the biological role of heavy metals. Formation * Hadhazy A. 2016,
Galactic 'gold mine' explains the origin of nature's heaviest elements
, ''Science Spotlights'', 10 May, accessed 11 July 2016 Uses * Koehler C. S. W. 2001,
, ''Chemistry Chronicles'', American Chemical Society, accessed 11 July 2016 * Morowitz N. 2006, "The heavy metals", ''Modern Marvels'', season 12, episode 14, History (U.S. TV channel), HistoryChannel.com * Öhrström L. 2014,
Tantalum oxide
, ''Chemistry World'', 24 September, accessed 4 October 2016. The author explains how tantalum(V) oxide banished brick-sized mobile phones. Also available as
podcast
atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
s. The criteria used, and whether metalloid
A metalloid is a type of chemical element which has a preponderance of material property, properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on ...
s are included, vary depending on the author and context. In metallurgy
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys.
Metallurgy encompasses both the sc ...
, for example, a heavy metal may be defined on the basis of density, whereas in physics the distinguishing criterion might be atomic number, while a chemist would likely be more concerned with chemical property, chemical behaviour. More specific definitions have been published, but none of these have been widely accepted. The definitions surveyed in this article encompass up to 96 out of the 118 known chemical element
A chemical element is a species of atoms that have a given number of protons in their atomic nucleus, nuclei, including the pure Chemical substance, substance consisting only of that species. Unlike chemical compounds, chemical elements canno ...
s; only mercury, lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, ...
and bismuth
Bismuth is a chemical element with the symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs ...
meet all of them. Despite this lack of agreement, the term (plural or singular) is widely used in science. A density of more than 5 g/cm3 is sometimes quoted as a commonly used criterion and is used in the body of this article.
The earliest known metals—common metals such as iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
, copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pink ...
, and tin, and precious metals such as silver
Silver is a chemical element with the symbol Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical ...
, gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile ...
, and platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Pla ...
—are heavy metals. From 1809 onward, light metal
A light metal is any metal of relatively low density. More specific definitions have been proposed; none have obtained widespread acceptance. Magnesium, aluminium and titanium are light metals of significant commercial importance. Their densities ...
s, such as magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
, aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
, and titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion i ...
, were discovered, as well as less well-known heavy metals including gallium
Gallium is a chemical element with the Symbol (chemistry), symbol Ga and atomic number 31. Discovered by France, French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in boron group, group 13 of the periodic table and is similar to ...
, thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes an ...
, and hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri M ...
.
Some heavy metals are either essential nutrients (typically iron, cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
, and zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
), or relatively harmless (such as ruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemical ...
, silver, and indium
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts ...
), but can be toxic in larger amounts or certain forms. Other heavy metals, such as cadmium
Cadmium is a chemical element with the Symbol (chemistry), symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12 element, group 12, zinc and mercury (element), mercury. Li ...
, mercury, and lead, are highly poisonous. Potential sources of heavy metal poisoning include mining
Mining is the extraction of valuable minerals or other geological materials from the Earth, usually from an ore body, lode, vein, seam, reef, or placer deposit. The exploitation of these deposits for raw material is based on the econom ...
, tailings
In mining, tailings are the materials left over after the process of separating the valuable fraction from the uneconomic fraction ( gangue) of an ore. Tailings are different to overburden, which is the waste rock or other material that ove ...
, industrial waste
Industrial waste is the waste produced by industrial activity which includes any material that is rendered useless during a manufacturing process such as that of factories, mills, and mining operations. Types of industrial waste include dirt an ...
, agricultural runoff, occupational exposure, paints and treated timber.
Physical and chemical characterisations of heavy metals need to be treated with caution, as the metals involved are not always consistently defined. As well as being relatively dense, heavy metals tend to be less reactive than lighter metals and have far fewer soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds la ...
s and hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. ...
s. While it is relatively easy to distinguish a heavy metal such as tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isol ...
from a lighter metal such as sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
, a few heavy metals, such as zinc, mercury, and lead, have some of the characteristics of lighter metals, and, lighter metals such as beryllium
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to for ...
, scandium
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the Lanthanides. It was discovered in ...
, and titanium, have some of the characteristics of heavier metals.
Heavy metals are relatively scarce in the Earth's crust
Earth's crust is Earth's thin outer shell of rock, referring to less than 1% of Earth's radius and volume. It is the top component of the lithosphere, a division of Earth's layers that includes the crust and the upper part of the mantle. The ...
but are present in many aspects of modern life. They are used in, for example, golf club
A golf club is a club used to hit a golf ball in a game of golf. Each club is composed of a shaft with a grip and a club head. Woods are mainly used for long-distance fairway or tee shots; irons, the most versatile class, are used for a variet ...
s, cars
A car or automobile is a motor vehicle with wheels. Most definitions of ''cars'' say that they run primarily on roads, Car seat, seat one to eight people, have four wheels, and mainly transport private transport#Personal transport, people in ...
, antiseptic
An antiseptic (from Greek ἀντί ''anti'', "against" and σηπτικός ''sēptikos'', "putrefactive") is an antimicrobial substance or compound that is applied to living tissue/skin to reduce the possibility of infection, sepsis, or putre ...
s, self-cleaning ovens, plastic
Plastics are a wide range of synthetic or semi-synthetic materials that use polymers as a main ingredient. Their plasticity makes it possible for plastics to be moulded, extruded or pressed into solid objects of various shapes. This adapta ...
s, solar panel
A solar cell panel, solar electric panel, photo-voltaic (PV) module, PV panel or solar panel is an assembly of photovoltaic solar cells mounted in a (usually rectangular) frame, and a neatly organised collection of PV panels is called a photo ...
s, mobile phone
A mobile phone, cellular phone, cell phone, cellphone, handphone, hand phone or pocket phone, sometimes shortened to simply mobile, cell, or just phone, is a portable telephone that can make and receive calls over a radio frequency link whi ...
s, and particle accelerator
A particle accelerator is a machine that uses electromagnetic fields to propel charged particles to very high speeds and energies, and to contain them in well-defined beams.
Large accelerators are used for fundamental research in particle ...
s.
Definitions
There is no widely agreed criterion-based definition of a heavy metal. Different meanings may be attached to the term, depending on the context. Inmetallurgy
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys.
Metallurgy encompasses both the sc ...
, for example, a heavy metal may be defined on the basis of density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematicall ...
, whereas in physics the distinguishing criterion might be atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
, and a chemist or biologist would likely be more concerned with chemical behaviour.
Density criteria range from above 3.5 g/cm3 to above 7 g/cm3. Atomic weight definitions can range from greater than sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
(atomic weight 22.98); greater than 40 (excluding s- and f-block
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-bl ...
metals, hence starting with scandium
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the Lanthanides. It was discovered in ...
); or more than 200, i.e. from mercury onwards. Atomic numbers of heavy metals are generally given as greater than 20 (calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar ...
); sometimes this is capped at 92 (uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
). Definitions based on atomic number have been criticised for including metals with low densities. For example, rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher ...
in group (column) 1 of the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
has an atomic number of 37 but a density of only 1.532 g/cm3, which is below the threshold figure used by other authors. The same problem may occur with atomic weight based definitions.
The United States Pharmacopeia
The ''United States Pharmacopeia'' (''USP'') is a pharmacopeia (compendium of drug information) for the United States published annually by the United States Pharmacopeial Convention (usually also called the USP), a nonprofit organization that ...
includes a test for heavy metals that involves precipitating metallic impurities as their coloured sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds la ...
s." In 1997, Stephen Hawkes, a chemistry professor writing in the context of fifty years' experience with the term, said it applied to "metals with insoluble sulfides and hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. ...
s, whose salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively c ...
produce colored solutions in water and whose complexes are usually colored". On the basis of the metals he had seen referred to as heavy metals, he suggested it would be useful to define them as (in general) all the metals in periodic table columns 3 to 16 that are in row 4 or greater, in other words, the transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
s and post-transition metal
The metallic elements in the periodic table located between the transition metals and the chemically weak nonmetallic metalloids have received many names in the literature, such as ''post-transition metals'', ''poor metals'', ''other metals'', ...
s. The lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yt ...
s satisfy Hawkes' three-part description; the status of the actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
s is not completely settled.
In biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
, heavy metals are sometimes defined—on the basis of the Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
(electronic pair acceptor) behaviour of their ions in aqueous solution—as class B and borderline metals. In this scheme, class A metal ions prefer oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
donors; class B ions prefer nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
or sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
donors; and borderline or ambivalent ions show either class A or B characteristics, depending on the circumstances. Class A metals, which tend to have low electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
and form bonds with large ionic character, are the alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
and alkaline earth
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are all ...
s, aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
, the group 3 metals, and the lanthanides and actinides. Class B metals, which tend to have higher electronegativity and form bonds with considerable covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
character, are mainly the heavier transition and post-transition metals. Borderline metals largely comprise the lighter transition and post-transition metals (plus arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, b ...
and antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient ti ...
). The distinction between the class A metals and the other two categories is sharp. A frequently cited proposal to use these classification categories instead of the more evocative name ''heavy metal'' has not been widely adopted.
List of heavy metals based on density
A density of more than 5 g/cm3 is sometimes mentioned as a common heavy metal defining factor and, in the absence of a unanimous definition, is used to populate this list and (unless otherwise stated) guide the remainder of the article. Metalloids meeting the applicable criteria–arsenic and antimony for example—are sometimes counted as heavy metals, particularly inenvironmental chemistry
Environmental chemistry is the scientific study of the chemical and biochemical phenomena that occur in natural places. It should not be confused with green chemistry, which seeks to reduce potential pollution at its source. It can be defined as ...
, as is the case here. Selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, ...
(density 4.8 g/cm3) is also included in the list. It falls marginally short of the density criterion and is less commonly recognised as a metalloid but has a waterborne chemistry similar in some respects to that of arsenic and antimony. Other metals sometimes classified or treated as "heavy" metals, such as beryllium
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to for ...
(density 1.8 g/cm3), aluminium (2.7 g/cm3), calcium (1.55 g/cm3), and barium (3.6 g/cm3) are here treated as light metal
A light metal is any metal of relatively low density. More specific definitions have been proposed; none have obtained widespread acceptance. Magnesium, aluminium and titanium are light metals of significant commercial importance. Their densities ...
s and, in general, are not further considered.
Origins and use of the term
The heaviness of naturally occurring metals such asgold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile ...
, copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pink ...
, and iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
may have been noticed in prehistory
Prehistory, also known as pre-literary history, is the period of human history between the use of the first stone tools by hominins 3.3 million years ago and the beginning of recorded history with the invention of writing systems. The use of ...
and, in light of their malleability
Ductility is a List of materials properties, mechanical property commonly described as a material's amenability to Drawing (manufacturing), drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a materia ...
, led to the first attempts to craft metal ornaments, tools, and weapons. All metals discovered from then until 1809 had relatively high densities; their heaviness was regarded as a singularly distinguishing criterion.
From 1809 onwards, light metals such as sodium, potassium, and strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is ex ...
were isolated. Their low densities challenged conventional wisdom and it was proposed to refer to them as ''metalloid
A metalloid is a type of chemical element which has a preponderance of material property, properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on ...
s'' (meaning "resembling metals in form or appearance"). This suggestion was ignored; the new elements came to be recognised as metals, and the term metalloid was then used to refer to nonmetallic elements and, later, elements that were hard to describe as either metals or nonmetals.
An early use of the term "heavy metal" dates from 1817, when the German chemist Leopold Gmelin
Leopold Gmelin (2 August 1788 – 13 April 1853) was a German chemist. Gmelin was a professor at the University of Heidelberg He worked on the red prussiate and created Gmelin's test, and wrote his ''Handbook of Chemistry'', which over successiv ...
divided the elements into nonmetals, light metals, and heavy metals. Light metals had densities of 0.860–5.0 g/cm3; heavy metals 5.308–22.000. The term later became associated with elements of high atomic weight or high atomic number. It is sometimes used interchangeably with the term ''heavy element''. For example, in discussing the history of nuclear chemistry
Nuclear chemistry is the sub-field of chemistry dealing with radioactivity, nuclear processes, and transformations in the nuclei of atoms, such as nuclear transmutation and nuclear properties.
It is the chemistry of radioactive elements such as t ...
, Magee notes that the actinides were once thought to represent a new heavy element transition group whereas Seaborg and co-workers "favoured ... a heavy metal rare-earth
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silve ...
like series ...". In astronomy
Astronomy () is a natural science that studies astronomical object, celestial objects and phenomena. It uses mathematics, physics, and chemistry in order to explain their origin and chronology of the Universe, evolution. Objects of interest ...
, however, a heavy element is any element heavier than hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
and helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
.
Criticism
In 2002, Scottish toxicologist John Duffus reviewed the definitions used over the previous 60 years and concluded they were so diverse as to effectively render the term meaningless. Along with this finding, the heavy metal status of some metals is occasionally challenged on the grounds that they are too light, or are involved in biological processes, or rarely constitute environmental hazards. Examples include scandium (too light);vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( pas ...
to zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
(biological processes); and rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isoto ...
, indium
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts ...
, and osmium
Osmium (from Greek grc, ὀσμή, osme, smell, label=none) is a chemical element with the symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a trace element in alloys, mos ...
(too rare).
Popularity
Despite its questionable meaning, the term ''heavy metal'' appears regularly in scientific literature. A 2010 study found that it had been increasingly used and seemed to have become part of the language of science. It is said to be an acceptable term, given its convenience and familiarity, as long as it is accompanied by a strict definition. The counterparts to the heavy metals, the ''light metals'', are alluded to by The Minerals, Metals and Materials Society as including "aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
, magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
, beryllium
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to for ...
, titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion i ...
, lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid el ...
, and other reactive metals."
Biological role
Trace amounts of some heavy metals, mostly in period 4, are required for certain biological processes. These areiron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
and copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pink ...
(oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
and electron transport
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples thi ...
); cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
( complex syntheses and cell metabolism); zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
(hydroxylation
In chemistry, hydroxylation can refer to:
*(i) most commonly, hydroxylation describes a chemical process that introduces a hydroxyl group () into an organic compound.
*(ii) the ''degree of hydroxylation'' refers to the number of OH groups in a ...
); vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( pas ...
and manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy use ...
( enzyme regulation or functioning); chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hardne ...
(glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using ...
utilisation); nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
(cell growth
Cell growth refers to an increase in the total mass of a cell, including both cytoplasmic, nuclear and organelle volume. Cell growth occurs when the overall rate of cellular biosynthesis (production of biomolecules or anabolism) is greater than ...
); arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, b ...
(metabolic growth in some animals and possibly in humans) and selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, ...
(antioxidant
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. This can lead to polymerization and other chain reactions. They are frequently added to industrial products, such as fuels and lubricant ...
functioning and hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are required ...
production). Periods 5 and 6 contain fewer essential heavy metals, consistent with the general pattern that heavier elements tend to be less abundant and that scarcer elements are less likely to be nutritionally essential. In period 5, molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
is required for the catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
of redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
reactions; cadmium
Cadmium is a chemical element with the Symbol (chemistry), symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12 element, group 12, zinc and mercury (element), mercury. Li ...
is used by some marine diatom
A diatom (Neo-Latin ''diatoma''), "a cutting through, a severance", from el, διάτομος, diátomos, "cut in half, divided equally" from el, διατέμνω, diatémno, "to cut in twain". is any member of a large group comprising sev ...
s for the same purpose; and tin may be required for growth in a few species. In period 6, tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isol ...
is required by some archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebac ...
and bacteria for metabolic processes. A deficiency of any of these period 4–6 essential heavy metals may increase susceptibility to heavy metal poisoning
A toxic heavy metal is any relatively dense metal or metalloid that is noted for its potential toxicity, especially in environmental contexts. The term has particular application to cadmium, mercury and lead, all of which appear in the World H ...
(conversely, an excess may also have adverse biological effects). An average 70 kg human body
The human body is the structure of a Human, human being. It is composed of many different types of Cell (biology), cells that together create Tissue (biology), tissues and subsequently organ systems. They ensure homeostasis and the life, viabi ...
is about 0.01% heavy metals (~7 g, equivalent to the weight of two dried peas, with iron at 4 g, zinc at 2.5 g, and lead at 0.12 g comprising the three main constituents), 2% light metals (~1.4 kg, the weight of a bottle of wine) and nearly 98% nonmetals (mostly water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
).
A few non-essential heavy metals have been observed to have biological effects. Gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group (aluminiu ...
, germanium (a metalloid), indium, and most lanthanides can stimulate metabolism, and titanium promotes growth in plants (though it is not always considered a heavy metal).
Toxicity
Heavy metals are often assumed to be highly toxic or damaging to the environment. Some are, while certain others are toxic only if taken in excess or encountered in certain forms. Inhalation of certain metals, either as fine dust or most commonly as fumes, can also result in a condition calledmetal fume fever
Metal fume fever, also known as brass founders' ague, brass shakes, zinc shakes, galvie flu, galvo poisoning, metal dust fever, welding shivers, or Monday morning fever, is an illness primarily caused by exposure to chemicals such as zinc oxide (Zn ...
.
Environmental heavy metals
Chromium, arsenic, cadmium, mercury, and lead have the greatest potential to cause harm on account of their extensive use, thetoxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ...
of some of their combined or elemental forms, and their widespread distribution in the environment. Hexavalent chromium
Hexavalent chromium (chromium(VI), Cr(VI), chromium 6) is chromium in any chemical compound that contains the element in the +6 oxidation state (thus hexavalent). Virtually all chromium ore is processed via hexavalent chromium, specifically the ...
, for example, is highly toxic as are mercury vapour and many mercury compounds. These five elements have a strong affinity for sulfur; in the human body they usually bind, via thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
groups (–SH), to enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s responsible for controlling the speed of metabolic reactions. The resulting sulfur-metal bonds inhibit the proper functioning of the enzymes involved; human health deteriorates, sometimes fatally. Chromium (in its hexavalent form) and arsenic are carcinogen
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substan ...
s; cadmium causes a degenerative bone disease; and mercury and lead damage the central nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all par ...
.
Chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hardne ...
crystalsand 1 cm3 cube
Arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but ...
, sealed in acontainer to stop tarnishing
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of ...
barand 1 cm3 cube
Mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
beingpoured into a
petri dish
A Petri dish (alternatively known as a Petri plate or cell-culture dish) is a shallow transparent lidded dish that biologists use to hold growth medium in which cells can be cultured,R. C. Dubey (2014): ''A Textbook Of Biotechnology For Class- ...
Oxidised
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, ...
nodules and 1 cm3 cube
tetraethyl lead
Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula Pb( C2H5)4. It is a fuel additive, first being mixed with gasoline beginning in the 1920s as a patented octane rating booster that al ...
, , it was used extensively in gasoline
Gasoline (; ) or petrol (; ) (see ) is a transparent, petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines (also known as petrol engines). It consists mostly of organic co ...
during the 1930s–1970s. Although the use of leaded gasoline was largely phased out in North America by 1996, soils next to roads built before this time retain high lead concentrations. Later research demonstrated a statistically significant correlation between the usage rate of leaded gasoline and violent crime in the United States; taking into account a 22-year time lag (for the average age of violent criminals), the violent crime curve virtually tracked the lead exposure curve.
Other heavy metals noted for their potentially hazardous nature, usually as toxic environmental pollutants, include manganese (central nervous system damage); cobalt and nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
(carcinogens); copper, zinc, selenium and silver
Silver is a chemical element with the symbol Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical ...
(endocrine
The endocrine system is a messenger system comprising feedback loops of the hormones released by internal glands of an organism directly into the circulatory system, regulating distant target organs. In vertebrates, the hypothalamus is the neu ...
disruption, congenital disorder
A birth defect, also known as a congenital disorder, is an abnormal condition that is present at birth regardless of its cause. Birth defects may result in disabilities that may be physical, intellectual, or developmental. The disabilities can ...
s, or general toxic effects in fish, plants, birds, or other aquatic organisms); tin, as organotin
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide (), discovered by ...
(central nervous system damage); antimony (a suspected carcinogen); and thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes an ...
(central nervous system damage).
Nutritionally essential heavy metals
Heavy metals essential for life can be toxic if taken in excess; some have notably toxic forms.Vanadium pentoxide
Vanadium(V) oxide (''vanadia'') is the inorganic compound with the formula V2 O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because o ...
(V2O5) is carcinogenic in animals and, when inhaled, causes DNA damage. The purple permanganate
A permanganate () is a chemical compound containing the manganate(VII) ion, , the conjugate base of permanganic acid. Because the manganese atom is in the +7 oxidation state, the permanganate(VII) ion is a strong oxidizing agent. The ion is a tra ...
ion MnO is a liver
The liver is a major Organ (anatomy), organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of proteins and biochemicals necessary for ...
and kidney
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; blood ...
poison. Ingesting more than 0.5 grams of iron can induce cardiac collapse; such overdoses most commonly occur in children and may result in death within 24 hours. Nickel carbonyl
Nickel carbonyl (IUPAC name: tetracarbonylnickel) is a nickel(0) organometallic compound with the formula Ni(CO)4. This colorless liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for producing very high-pu ...
(Ni(CO)4), at 30 parts per million, can cause respiratory failure, brain damage and death. Imbibing a gram or more of copper sulfate Copper sulfate may refer to:
* Copper(II) sulfate, CuSO4, a common compound used as a fungicide and herbicide
* Copper(I) sulfate
Copper(I) sulfate, also known as cuprous sulfate, is an inorganic compound with the chemical formula Cu2 SO4. It ...
(CuSO4) can be fatal; survivors may be left with major organ damage. More than five milligrams of selenium is highly toxic; this is roughly ten times the 0.45 milligram recommended maximum daily intake; long-term poisoning can have paralytic effects.
Other heavy metals
A few other non-essential heavy metals have one or more toxic forms. Kidney failure and fatalities have been recorded arising from the ingestion of germanium dietary supplements (~15 to 300 g in total consumed over a period of two months to three years). Exposure toosmium tetroxide
Osmium tetroxide (also osmium(VIII) oxide) is the chemical compound with the formula OsO4. The compound is noteworthy for its many uses, despite its toxicity and the rarity of osmium. It also has a number of unusual properties, one being that the ...
(OsO4) may cause permanent eye damage and can lead to respiratory failure and death. Indium salts are toxic if more than few milligrams are ingested and will affect the kidneys, liver, and heart. Cisplatin
Cisplatin is a chemotherapy medication used to treat a number of cancers. These include testicular cancer, ovarian cancer, cervical cancer, breast cancer, bladder cancer, head and neck cancer, esophageal cancer, lung cancer, mesothelioma, br ...
(PtCl2(NH3)2), which is an important drug used to kill cancer cells, is also a kidney and nerve poison. Bismuth
Bismuth is a chemical element with the Symbol (chemistry), symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental ...
compounds can cause liver damage if taken in excess; insoluble uranium compounds, as well as the dangerous radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or through a material medium. This includes:
* ''electromagnetic radiation'', such as radio waves, microwaves, infrared, visi ...
they emit, can cause permanent kidney damage.
Exposure sources
Heavy metals can degrade air, water, and soil quality, and subsequently cause health issues in plants, animals, and people, when they become concentrated as a result of industrial activities. Common sources of heavy metals in this context include mining and industrial wastes; vehicle emissions; motor oil; fuels used by ships and heavy machineries; construction works; fertilisers; pesticides; paints; dyes and pigments; renovation; illegal depositing of construction and demolition waste; open-top roll-off dumpster; welding, brazing and soldering; glassworking; concrete works; roadworks; use of recycled materials; DIY Metal Projects; burning ofjoss paper
Joss paper, also known as incense papers, are papercrafts or sheets of paper made into burnt offerings common in Chinese ancestral worship (such as the veneration of the deceased family members and relatives on holidays and special occasions). Wo ...
; open burning of waste in rural area; contaminated ventilation system; food contaminated by the environment or by the packaging; armaments; lead–acid batteries; electronic waste
Electronic waste or e-waste describes discarded electrical or electronic devices. Used electronics which are destined for refurbishment, reuse, resale, salvage recycling through material recovery, or disposal are also considered e-waste. Informa ...
recycling yard; and treated timber; aging water supply infrastructure; and microplastics
Microplastics are fragments of any type of plastic less than in length, according to the U.S. National Oceanic and Atmospheric Administration (NOAA) and the European Chemicals Agency. They cause pollution by entering natural ecosystems from a v ...
floating in the world's oceans. Recent examples of heavy metal contamination and health risks include the occurrence of Minamata disease
Minamata disease is a neurological disease caused by severe mercury poisoning. Signs and symptoms include ataxia, numbness in the hands and feet, general muscle weakness, loss of peripheral vision, and damage to hearing and speech. In extrem ...
, in Japan (1932–1968; lawsuits ongoing as of 2016); the Bento Rodrigues dam disaster
The Mariana dam disaster, also known as the Bento Rodrigues or Samarco dam disaster, occurred on 5 November 2015, when the Fundão tailings dam at the Germano iron ore mine of the Samarco Mariana Mining Complex near Mariana, Minas Gerais, Braz ...
in Brazil, high levels of lead in drinking water supplied to the residents of Flint
Flint, occasionally flintstone, is a sedimentary cryptocrystalline form of the mineral quartz, categorized as the variety of chert that occurs in chalk or marly limestone. Flint was widely used historically to make stone tools and start fir ...
, Michigan, in the north-east of the United States and 2015 Hong Kong heavy metal in drinking water incidents.
Formation, abundance, occurrence, and extraction
Heavy metals up to the vicinity of iron (in the periodic table) are largely made viastellar nucleosynthesis
Stellar nucleosynthesis is the creation (nucleosynthesis) of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a ...
. In this process, lighter elements from hydrogen to silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic tab ...
undergo successive fusion
Fusion, or synthesis, is the process of combining two or more distinct entities into a new whole.
Fusion may also refer to:
Science and technology Physics
*Nuclear fusion, multiple atomic nuclei combining to form one or more different atomic nucl ...
reactions inside stars, releasing light and heat and forming heavier elements with higher atomic numbers.
Heavier heavy metals are not usually formed this way since fusion reactions involving such nuclei would consume rather than release energy. Rather, they are largely synthesised (from elements with a lower atomic number) by neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, ...
, with the two main modes of this repetitive capture being the s-process
The slow neutron-capture process, or ''s''-process, is a series of reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The ''s''-process is responsible for the creation (nucleosynthesis) of approximat ...
and the r-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for the creation of approximately half of the atomic nuclei heavier than iron, the "heavy elements", ...
. In the s-process ("s" stands for "slow"), singular captures are separated by years or decades, allowing the less stable nuclei to beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
, while in the r-process ("rapid"), captures happen faster than nuclei can decay. Therefore, the s-process takes a more or less clear path: for example, stable cadmium-110 nuclei are successively bombarded by free neutrons inside a star until they form cadmium-115 nuclei which are unstable and decay to form indium-115 (which is nearly stable, with a half-life times the age of the universe). These nuclei capture neutrons and form indium-116, which is unstable, and decays to form tin-116, and so on. In contrast, there is no such path in the r-process. The s-process stops at bismuth due to the short half-lives of the next two elements, polonium and astatine, which decay to bismuth or lead. The r-process is so fast it can skip this zone of instability and go on to create heavier elements such as thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high me ...
and uranium.
Heavy metals condense in planets as a result of stellar evolution and destruction processes. Stars lose much of their mass when it is ejected
Ejection or Eject may refer to:
* Ejection (sports), the act of officially removing someone from a game
* Eject (''Transformers''), a fictional character from ''The Transformers'' television series
* "Eject" (song), 1993 rap rock single by Sense ...
late in their lifetimes, and sometimes thereafter as a result of a neutron star
A neutron star is the collapsed core of a massive supergiant star, which had a total mass of between 10 and 25 solar masses, possibly more if the star was especially metal-rich. Except for black holes and some hypothetical objects (e.g. white ...
merger, thereby increasing the abundance of elements heavier than helium in the interstellar medium
In astronomy, the interstellar medium is the matter and radiation that exist in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstella ...
. When gravitational attraction causes this matter to coalesce and collapse, new stars and planets are formed.
The Earth's crust is made of approximately 5% of heavy metals by weight, with iron comprising 95% of this quantity. Light metals (~20%) and nonmetals (~75%) make up the other 95% of the crust. Despite their overall scarcity, heavy metals can become concentrated in economically extractable quantities as a result of mountain building
Mountain formation refers to the geological processes that underlie the formation of mountains. These processes are associated with large-scale movements of the Earth's crust (tectonic plates). Folding, faulting, volcanic activity, igneous int ...
, erosion
Erosion is the action of surface processes (such as water flow or wind) that removes soil, rock, or dissolved material from one location on the Earth's crust, and then transports it to another location where it is deposited. Erosion is distin ...
, or other geological processes
Geology () is a branch of natural science concerned with Earth and other astronomical objects, the features or rocks of which it is composed, and the processes by which they change over time. Modern geology significantly overlaps all other Eart ...
.
Heavy metals are found primarily as lithophiles
Lithophiles are micro-organisms that can live within the pore interstices of sedimentary and even fractured igneous rocks to depths of several kilometers.
Some are known to live on surface rocks, and make use of photosynthesis for energy.
Thos ...
(rock-loving) or chalcophiles (ore-loving). Lithophile heavy metals are mainly f-block elements and the more reactive of the d-block
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-blo ...
elements. They have a strong affinity for oxygen and mostly exist as relatively low density silicate minerals
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.
In mineralogy, silica (silicon dioxide, ) is usually con ...
. Chalcophile heavy metals are mainly the less reactive d-block elements, and period 4–6 p-block
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-blo ...
metals and metalloids. They are usually found in (insoluble) sulfide minerals
The sulfide minerals are a class of minerals containing sulfide (S2−) or disulfide (S22−) as the major anion. Some sulfide minerals are economically important as metal ores. The sulfide class also includes the selenides, the tellurides, the ...
. Being denser than the lithophiles, hence sinking lower into the crust at the time of its solidification, the chalcophiles tend to be less abundant than the lithophiles.
In contrast, gold is a siderophile, or iron-loving element. It does not readily form compounds with either oxygen or sulfur. At the time of the Earth's formation, and as the most noble
A noble is a member of the nobility.
Noble may also refer to:
Places Antarctica
* Noble Glacier, King George Island
* Noble Nunatak, Marie Byrd Land
* Noble Peak, Wiencke Island
* Noble Rocks, Graham Land
Australia
* Noble Island, Great B ...
(inert) of metals, gold sank into the core
Core or cores may refer to:
Science and technology
* Core (anatomy), everything except the appendages
* Core (manufacturing), used in casting and molding
* Core (optical fiber), the signal-carrying portion of an optical fiber
* Core, the centra ...
due to its tendency to form high-density metallic alloys. Consequently, it is a relatively rare metal. Some other (less) noble heavy metals—molybdenum, rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
, the platinum group metal
The platinum-group metals (abbreviated as the PGMs; alternatively, the platinoids, platinides, platidises, platinum group, platinum metals, platinum family or platinum-group elements (PGEs)) are six noble, precious metallic elements clustered t ...
s (ruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemical ...
, rhodium, palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
, osmium, iridium
Iridium is a chemical element with the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal (after osmium) with a density of ...
, and platinum), germanium, and tin—can be counted as siderophiles but only in terms of their primary occurrence in the Earth (core, mantle and crust), rather the crust. These metals otherwise occur in the crust, in small quantities, chiefly as chalcophiles (less so in their native form).
Concentrations of heavy metals below the crust are generally higher, with most being found in the largely iron-silicon-nickel core. Platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
, for example, comprises approximately 1 part per billion of the crust whereas its concentration in the core is thought to be nearly 6,000 times higher. Recent speculation suggests that uranium (and thorium) in the core may generate a substantial amount of the heat that drives plate tectonics
Plate tectonics (from the la, label=Late Latin, tectonicus, from the grc, τεκτονικός, lit=pertaining to building) is the generally accepted scientific theory that considers the Earth's lithosphere to comprise a number of large ...
and (ultimately) sustains the Earth's magnetic field
Earth's magnetic field, also known as the geomagnetic field, is the magnetic field that extends from Earth's interior out into space, where it interacts with the solar wind, a stream of charged particles emanating from the Sun. The magnetic f ...
.
Broadly speaking, and with some exceptions, lithophile heavy metals can be extracted from their ores by electrical
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by ...
or chemical treatments, while chalcophile heavy metals are obtained by roasting
Roasting is a cooking method that uses dry heat where hot air covers the food, cooking it evenly on all sides with temperatures of at least from an open flame, oven, or other heat source. Roasting can enhance the flavor through caramelization ...
their sulphide ores to yield the corresponding oxides, and then heating these to obtain the raw metals. Radium occurs in quantities too small to be economically mined and is instead obtained from spent nuclear fuel
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergoing ...
s. The chalcophile platinum group metals (PGM) mainly occur in small (mixed) quantities with other chalcophile ores. The ores involved need to be smelted
Smelting is a process of applying heat to ore, to extract a base metal. It is a form of extractive metallurgy. It is used to extract many metals from their ores, including silver, iron, copper, and other base metals. Smelting uses heat and a c ...
, roasted, and then leached with sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
to produce a residue of PGM. This is chemically refined to obtain the individual metals in their pure forms. Compared to other metals, PGM are expensive due to their scarcity and high production costs.
Gold, a siderophile, is most commonly recovered by dissolving the ores in which it is found in a cyanide solution. The gold forms a dicyanoaurate(I), for example: 2 Au + H2O +½ O2 + 4 KCN → 2 K u(CN)2+ 2 KOH. Zinc is added to the mix and, being more reactive
Reactive may refer to:
*Generally, capable of having a reaction (disambiguation)
*An adjective abbreviation denoting a bowling ball coverstock made of reactive resin
*Reactivity (chemistry)
*Reactive mind
*Reactive programming
See also
*Reactanc ...
than gold, displaces the gold: 2 K u(CN)2+ Zn → K2 n(CN)4+ 2 Au. The gold precipitates out of solution as a sludge, and is filtered off and melted.
Properties compared with light metals
Some general physical and chemical properties of light and heavy metals are summarised in the table. The comparison should be treated with caution since the terms light metal and heavy metal are not always consistently defined. Also the physical properties of hardness and tensile strength can vary widely depending on purity, grain size and pre-treatment. These properties make it relatively easy to distinguish a light metal like sodium from a heavy metal like tungsten, but the differences become less clear at the boundaries. Light structural metals like beryllium, scandium, and titanium have some of the characteristics of heavy metals, such as higher melting points; post-transition heavy metals like zinc, cadmium, and lead have some of the characteristics of light metals, such as being relatively soft, having lower melting points, and forming mainly colourless complexes.Uses
Heavy metals are present in nearly all aspects of modern life. Iron may be the most common as it accounts for 90% of all refined metals. Platinum may be the most ubiquitous given it is said to be found in, or used to produce, 20% of all consumer goods. Some common uses of heavy metals depend on the general characteristics of metals such aselectrical conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allow ...
and reflectivity
The reflectance of the surface of a material is its effectiveness in reflecting radiant energy. It is the fraction of incident electromagnetic power that is reflected at the boundary. Reflectance is a component of the response of the electronic ...
or the general characteristics of heavy metals such as density, strength, and durability. Other uses depend on the characteristics of the specific element, such as their biological role as nutrients or poisons or some other specific atomic properties. Examples of such atomic properties include: partly filled d- or f- orbitals (in many of the transition, lanthanide, and actinide heavy metals) that enable the formation of coloured compounds; the capacity of most heavy metal ions (such as platinum, cerium or bismuth) to exist in different oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
s and therefore act as catalysts; poorly overlapping 3d or 4f orbitals (in iron, cobalt, and nickel, or the lanthanide heavy metals from europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lanth ...
through thulium
Thulium is a chemical element with the symbol Tm and atomic number 69. It is the thirteenth and third-last element in the lanthanide series. Like the other lanthanides, the most common oxidation state is +3, seen in its oxide, halides and other c ...
) that give rise to magnetic effects; and high atomic numbers and electron densities that underpin their nuclear science applications. Typical uses of heavy metals can be broadly grouped into the following six categories.
Weight- or density-based
 Some uses of heavy metals, including in sport,
Some uses of heavy metals, including in sport, mechanical engineering
Mechanical engineering is the study of physical machines that may involve force and movement. It is an engineering branch that combines engineering physics and mathematics principles with materials science, to design, analyze, manufacture, and ...
, military ordnance, and nuclear science
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.
Nuclear physics should not be confused with atomic physics, which studies the ...
, take advantage of their relatively high densities. In underwater diving
Underwater diving, as a human activity, is the practice of descending below the water's surface to interact with the environment. It is also often referred to as diving, an ambiguous term with several possible meanings, depending on context ...
, lead is used as a ballast
Ballast is material that is used to provide stability to a vehicle or structure. Ballast, other than cargo, may be placed in a vehicle, often a ship or the gondola of a balloon or airship, to provide stability. A compartment within a boat, ship, ...
; in handicap horse racing each horse must carry a specified lead weight, based on factors including past performance, so as to equalize the chances of the various competitors. In golf
Golf is a club-and-ball sport in which players use various clubs to hit balls into a series of holes on a course in as few strokes as possible.
Golf, unlike most ball games, cannot and does not use a standardized playing area, and coping wi ...
, tungsten, brass
Brass is an alloy of copper (Cu) and zinc (Zn), in proportions which can be varied to achieve different mechanical, electrical, and chemical properties. It is a substitutional alloy: atoms of the two constituents may replace each other with ...
, or copper inserts in fairway clubs and irons lower the centre of gravity of the club making it easier to get the ball into the air; and golf balls with tungsten cores are claimed to have better flight characteristics. In fly fishing
Fly fishing is an angling method that uses a light-weight lure—called an artificial fly—to catch fish. The fly is cast using a fly rod, reel, and specialized weighted line. The light weight requires casting techniques significantly diffe ...
, sinking fly lines have a PVC coating embedded with tungsten powder, so that they sink at the required rate. In track and field
Track and field is a sport that includes athletic contests based on running, jumping, and throwing skills. The name is derived from where the sport takes place, a running track and a grass field for the throwing and some of the jumping events ...
sport, steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant ty ...
balls used in the hammer throw
The hammer throw is one of the four throwing events in regular track and field competitions, along with the discus throw, shot put and javelin.
The "hammer" used in this sport is not like any of the tools also called by that name. It consis ...
and shot put
The shot put is a track and field event involving "putting" (throwing) a heavy spherical ball—the ''shot''—as far as possible. The shot put competition for men has been a part of the modern Olympics since their revival in 1896, and women's ...
events are filled with lead in order to attain the minimum weight required under international rules. Tungsten was used in hammer throw balls at least up to 1980; the minimum size of the ball was increased in 1981 to eliminate the need for what was, at that time, an expensive metal (triple the cost of other hammers) not generally available in all countries. Tungsten hammers were so dense that they penetrated too deeply into the turf.
In mechanical engineering, heavy metals are used for ballast in boats, aeroplanes, and motor vehicles; or in tire balance, balance weights on wheels and crankshafts, gyroscopes, and propellers, and centrifugal clutches, in situations requiring maximum weight in minimum space (for example in movement (clockwork), watch movements).
In military ordnance, tungsten or uranium is used in Chobham armour#Heavy metal modules, armour plating and kinetic energy penetrator, armour piercing projectiles, as well as in Nuclear weapon design, nuclear weapons to increase efficiency (by neutron reflector, reflecting neutrons and momentarily delaying the expansion of reacting materials). In the 1970s, tantalum was found to be more effective than copper in shaped charge and explosively formed penetrator, explosively formed anti-armour weapons on account of its higher density, allowing greater force concentration, and better deformability. Less-toxic heavy metals, such as copper, tin, tungsten, and bismuth, and probably manganese (as well as boron, a metalloid), have replaced lead and antimony in the green bullets used by some armies and in some recreational shooting munitions. Doubts have been raised about the safety (or environmentally friendly, green credentials) of tungsten.
Because denser materials absorb more radioactive emissions than lighter ones, heavy metals are useful for radiation protection, radiation shielding and to collimator#Neutron, X-ray, and gamma ray collimators, focus radiation beams in linear accelerators and radiotherapy applications.
Strength- or durability-based
 The strength or durability of heavy metals such as chromium, iron, nickel, copper, zinc, molybdenum, tin, tungsten, and lead, as well as their alloys, makes them useful for the manufacture of artefacts such as tools, machinery, domestic appliances, appliances, utensils, pipes, railroad tracks, buildings and bridges, automobiles, locks, furniture, ships, planes, coinage and jewellery. They are also used as alloying additives for enhancing the properties of other metals. Of the two dozen elements that have been used in the world's monetised coinage only two, carbon and aluminium, are not heavy metals. Gold, silver, and platinum are used in jewellery as are (for example) nickel, copper, indium, and cobalt in colored gold, coloured gold. Costume jewelry, Low-cost jewellery and toy#Safety regulations, children's toys may be made, to a significant degree, of heavy metals such as chromium, nickel, cadmium, or lead.
Copper, zinc, tin, and lead are mechanically weaker metals but have useful corrosion prevention properties. While each of them will react with air, the resulting patinas of either various copper salts, zinc carbonate, tin dioxide, tin oxide, or a mixture of lead(II) oxide, lead oxide, lead carbonate, carbonate, and Lead sulfate, sulfate, confer valuable passivation (chemistry), protective properties. Copper and lead are therefore used, for example, as roofing materials; zinc acts as an anti-corrosion agent in galvanised steel; and tin serves a similar purpose on steel cans.
The workability and corrosion resistance of iron and chromium are increased by adding gadolinium; the creep (deformation), creep resistance of nickel is improved with the addition of thorium. Tellurium is added to copper (tellurium copper) and steel alloys to improve their machinability; and to lead to make it harder and more acid-resistant.
The strength or durability of heavy metals such as chromium, iron, nickel, copper, zinc, molybdenum, tin, tungsten, and lead, as well as their alloys, makes them useful for the manufacture of artefacts such as tools, machinery, domestic appliances, appliances, utensils, pipes, railroad tracks, buildings and bridges, automobiles, locks, furniture, ships, planes, coinage and jewellery. They are also used as alloying additives for enhancing the properties of other metals. Of the two dozen elements that have been used in the world's monetised coinage only two, carbon and aluminium, are not heavy metals. Gold, silver, and platinum are used in jewellery as are (for example) nickel, copper, indium, and cobalt in colored gold, coloured gold. Costume jewelry, Low-cost jewellery and toy#Safety regulations, children's toys may be made, to a significant degree, of heavy metals such as chromium, nickel, cadmium, or lead.
Copper, zinc, tin, and lead are mechanically weaker metals but have useful corrosion prevention properties. While each of them will react with air, the resulting patinas of either various copper salts, zinc carbonate, tin dioxide, tin oxide, or a mixture of lead(II) oxide, lead oxide, lead carbonate, carbonate, and Lead sulfate, sulfate, confer valuable passivation (chemistry), protective properties. Copper and lead are therefore used, for example, as roofing materials; zinc acts as an anti-corrosion agent in galvanised steel; and tin serves a similar purpose on steel cans.
The workability and corrosion resistance of iron and chromium are increased by adding gadolinium; the creep (deformation), creep resistance of nickel is improved with the addition of thorium. Tellurium is added to copper (tellurium copper) and steel alloys to improve their machinability; and to lead to make it harder and more acid-resistant.
Biological and chemical
 The biocide, biocidal effects of oligodynamic effect, some heavy metals have been known since antiquity. Platinum, osmium, copper, ruthenium, and other heavy metals, including arsenic, are used in anti-cancer treatments, or have shown potential. Antimony (anti-protozoal), bismuth (antiulcer agent, anti-ulcer), gold (arthritis#Medications, anti-arthritic), and iron (anti-malarial medication, anti-malarial) are also important in medicine. Copper, zinc, silver, gold, or mercury are used in
The biocide, biocidal effects of oligodynamic effect, some heavy metals have been known since antiquity. Platinum, osmium, copper, ruthenium, and other heavy metals, including arsenic, are used in anti-cancer treatments, or have shown potential. Antimony (anti-protozoal), bismuth (antiulcer agent, anti-ulcer), gold (arthritis#Medications, anti-arthritic), and iron (anti-malarial medication, anti-malarial) are also important in medicine. Copper, zinc, silver, gold, or mercury are used in antiseptic
An antiseptic (from Greek ἀντί ''anti'', "against" and σηπτικός ''sēptikos'', "putrefactive") is an antimicrobial substance or compound that is applied to living tissue/skin to reduce the possibility of infection, sepsis, or putre ...
formulations; small amounts of some heavy metals are used to control algal growth in, for example, cooling towers. Depending on their intended use as fertilisers or biocides, agrochemicals may contain heavy metals such as chromium, cobalt, nickel, copper, zinc, arsenic, cadmium, mercury, or lead.
Selected heavy metals are used as catalysts in fuel processing (rhenium, for example), synthetic rubber and fibre production (bismuth), catalytic converter#Construction, emission control devices (palladium), and in self-cleaning ovens (where cerium oxide, cerium(IV) oxide in the walls of such ovens helps redox, oxidise carbon-based cooking residues). In soap chemistry, heavy metals form insoluble soaps that are used in grease (lubricant), lubricating greases, paint dryers, and fungicides (apart from lithium, the alkali metals and the ammonium ion form soluble soaps).
Colouring and optics
plastic
Plastics are a wide range of synthetic or semi-synthetic materials that use polymers as a main ingredient. Their plasticity makes it possible for plastics to be moulded, extruded or pressed into solid objects of various shapes. This adapta ...
s are commonly produced by the inclusion of heavy metals (or their compounds) such as chromium, manganese, cobalt, copper, zinc, selenium, zirconium, molybdenum, silver, tin, praseodymium, neodymium, erbium, tungsten, iridium, gold, lead, or uranium. Tattoo inks may contain heavy metals, such as chromium, cobalt, nickel, and copper. The high reflectivity of some heavy metals is important in the construction of mirrors, including precision astronomical instruments. Headlight reflectors rely on the excellent reflectivity of a thin film of rhodium.
Electronics, magnets, and lighting
 Heavy metals or their compounds can be found in electronic components, electrodes, and electrical wiring, wiring and
Heavy metals or their compounds can be found in electronic components, electrodes, and electrical wiring, wiring and solar panel
A solar cell panel, solar electric panel, photo-voltaic (PV) module, PV panel or solar panel is an assembly of photovoltaic solar cells mounted in a (usually rectangular) frame, and a neatly organised collection of PV panels is called a photo ...
s where they may be used as either conductors, semiconductors, or insulators. Molybdenum powder is used in circuit board inks. Ruthenium(IV) oxide coated titanium anodes are used for the industrial production of chlorine. Home electrical systems, for the most part, are wired with copper wire for its good conducting properties. Silver and gold are used in electrical and electronic devices, particularly in contact switches, as a result of their high electrical conductivity and capacity to resist or minimise the formation of impurities on their surfaces. The semiconductors cadmium telluride and gallium arsenide are used to make solar panels. Hafnium oxide, an insulator, is used as a voltage controller in microchips; tantalum oxide, another insulator, is used in capacitors in mobile phone
A mobile phone, cellular phone, cell phone, cellphone, handphone, hand phone or pocket phone, sometimes shortened to simply mobile, cell, or just phone, is a portable telephone that can make and receive calls over a radio frequency link whi ...
s. Heavy metals have been used in batteries for over 200 years, at least since Alessandro Volta, Volta invented his copper and silver voltaic pile in 1800. Promethium, lanthanum, and mercury are further examples found in, respectively, atomic battery, atomic, nickel-metal hydride battery, nickel-metal hydride, and button cell batteries.
Magnets are made of heavy metals such as manganese, iron, cobalt, nickel, niobium, bismuth, praseodymium, neodymium, gadolinium, and dysprosium. Neodymium magnets are the strongest type of magnet#Types of permanent magnets, permanent magnet commercially available. They are key components of, for example, car door locks, starter motors, fuel pumps, and power windows.
Heavy metals are used in lighting, lasers, and light-emitting diodes (LEDs). Flat panel displays incorporate a thin film of electrically conducting indium tin oxide. Fluorescent lighting relies on mercury vapour for its operation. Ruby lasers generate deep red beams by exciting chromium atoms; the lanthanides are also extensively employed in lasers. Gallium, indium, and arsenic; and copper, iridium, and platinum are used in LEDs (the latter three in organic LEDs).
Nuclear
 Niche uses of heavy metals with high atomic numbers occur in diagnostic imaging, transmission electron microscopy, electron microscopy, and nuclear science. In diagnostic imaging, heavy metals such as cobalt or tungsten make up the anode materials found in x-ray tubes. In electron microscopy, heavy metals such as lead, gold, palladium, platinum, or uranium are used to make conductive coatings and to introduce electron density into biological specimens by staining, negative staining, or evaporation (deposition), vacuum deposition. In nuclear science, nuclei of heavy metals such as chromium, iron, or zinc are sometimes fired at other heavy metal targets to produce transuranium element#Super-heavy elements, superheavy elements; heavy metals are also employed as spallation#Nuclear spallation, spallation targets for the production of neutrons or radioisotopes such as astatine (using lead, bismuth, thorium, or uranium in the latter case).
Niche uses of heavy metals with high atomic numbers occur in diagnostic imaging, transmission electron microscopy, electron microscopy, and nuclear science. In diagnostic imaging, heavy metals such as cobalt or tungsten make up the anode materials found in x-ray tubes. In electron microscopy, heavy metals such as lead, gold, palladium, platinum, or uranium are used to make conductive coatings and to introduce electron density into biological specimens by staining, negative staining, or evaporation (deposition), vacuum deposition. In nuclear science, nuclei of heavy metals such as chromium, iron, or zinc are sometimes fired at other heavy metal targets to produce transuranium element#Super-heavy elements, superheavy elements; heavy metals are also employed as spallation#Nuclear spallation, spallation targets for the production of neutrons or radioisotopes such as astatine (using lead, bismuth, thorium, or uranium in the latter case).
Notes
Sources
Citations
References
* Ahrland S., Jan-Olov Liljenzin, Liljenzin J. O. & Rydberg J. 1973, "Solution chemistry," in J. C. Bailar & Aubrey Trotman-Dickenson, A. F. Trotman-Dickenson (eds), ''Comprehensive Inorganic Chemistry'', vol. 5, The Actinides, Pergamon Press, Oxford. * Albutt M. & Dell R. 1963, ''The nitrites and sulphides of uranium, thorium and plutonium: A review of present knowledge'', UK Atomic Energy Authority Research Group, Atomic Energy Research Establishment, Harwell, Berkshire. * Alves A. K., Berutti, F. A. & Sánche, F. A. L. 2012, "Nanomaterials and catalysis", in C. P. Bergmann & M. J. de Andrade (ads), ''Nanonstructured Materials for Engineering Applications'', Springer-Verlag, Berlin, . * Amasawa E., Yi Teah H., Yu Ting Khew, J., Ikeda I. & Onuki M. 2016, "Drawing Lessons from the Minamata Incident for the General Public: Exercise on Resilience, Minamata Unit AY2014", in M. Esteban, T. Akiyama, C. Chen, I. Ikea, T. Mino (eds), ''Sustainability Science: Field Methods and Exercises'', Springer International, Switzerland, pp. 93–116, . * Ariel E., Barta J. & Brandon D. 1973, "Preparation and properties of heavy metals", ''Powder Metallurgy International'', vol. 5, no. 3, pp. 126–129. * Atlas R. M. 1986, ''Basic and Practical Microbiology'', Macmillan Publishers, Macmillan Publishing Company, New York, . * Australian Government 2016,National Pollutant Inventory
', Department of the Environment and Energy, accessed 16 August 2016. * Baird C. & Cann M. 2012, ''Environmental Chemistry'', 5th ed., W. H. Freeman and Company, New York, . * Baldwin D. R. & Marshall W. J. 1999, "Heavy metal poisoning and its laboratory investigation", ''Annals of Clinical Biochemistry'', vol. 36, no. 3, pp. 267–300, . * Ball J. L., Moore A. D. & Turner S. 2008, ''Ball and Moore's Essential Physics for Radiographers,'' 4th ed., Blackwell Publishing, Chichester, . * Bánfalvi G. 2011, "Heavy metals, trace elements and their cellular effects", in G. Bánfalvi (ed.), ''Cellular Effects of Heavy Metals'', Axel Springer SE, Springer, Dordrecht, pp. 3–28, . * Baranoff E. 2015, "First-row transition metal complexes for the conversion of light into electricity and electricity into light", in W-Y Wong (ed.), ''Organometallics and Related Molecules for Energy Conversion'', Springer, Heidelberg, pp. 61–90, . * Berea E., Rodriguez-lbelo M. & Navarro J. A. R. 2016, "Platinum Group Metal—Organic frameworks" in S. Kaskel (ed.), ''The Chemistry of Metal-Organic Frameworks: Synthesis, Characterisation, and Applications'', vol. 2, Wiley-VCH Weinheim, pp. 203–230, . * Berger A. J. & Bruning N. 1979, ''Lady Luck's Companion: How to Play ... How to Enjoy ... How to Bet ... How to Win'', Harper & Row, New York, . * Berry L. G. & Mason B. 1959, ''Mineralogy: Concepts, Descriptions, Determinations'', W. H. Freeman and Company, San Francisco. * Biddle H. C. & Bush G. L 1949, ''Chemistry Today'', Rand McNally, Chicago. * Bonchev D. & Kamenska V. 1981, "Predicting the properties of the 113–120 transactinide elements", ''The Journal of Physical Chemistry'', vo. 85, no. 9, pp. 1177–1186, . * Bonetti A., Leone R., Muggia F. & Howell S. B. (eds) 2009, ''Platinum and Other Heavy Metal Compounds in Cancer Chemotherapy: Molecular Mechanisms and Clinical Applications'', Humana Press, New York, . * Booth H. S. 1957, ''Inorganic Syntheses'', vol. 5, McGraw-Hill, New York. * Bradl H. E. 2005, "Sources and origins of heavy metals", in Bradl H. E. (ed.), ''Heavy Metals in the Environment: Origin, Interaction and Remediation'', Elsevier, Amsterdam, . * Brady J. E. & Holum J. R. 1995, ''Chemistry: The Study of Matter and its Changes'', 2nd ed., John Wiley & Sons, New York, . * Brephohl E. & Tim McCreight, McCreight T. (ed) 2001, ''The Theory and Practice of Goldsmithing,'' C. Lewton-Brain trans., Brynmorgen Press, Portland, Maine, . * Brown I. 1987, "Astatine: Its organonuclear chemistry and biomedical applications," in H. J. Emeléus & A. G. Sharpe (eds), ''Advances in Inorganic Chemistry'', vol. 31, Academic Press, Orlando, pp. 43–88, . * Bryson R. M. & Hammond C. 2005, "Generic methodologies for nanotechnology: Characterisation"', in R. Kelsall, I. W. Hamley & M. Geoghegan, ''Nanoscale Science and Technology'', John Wiley & Sons, Chichester, pp. 56–129, . * Burkett B. 2010, ''Sport Mechanics for Coaches'', 3rd ed., Human Kinetics, Champaign, Illinois, . * Casey C. 1993, "Restructuring work: New work and new workers in post-industrial production", in R. P. Coulter & I. F. Goodson (eds), ''Rethinking Vocationalism: Whose Work/life is it?'', Our Schools/Our Selves Education Foundation, Toronto, . * Chakhmouradian A.R., Smith M. P. & Kynicky J. 2015, "From "strategic" tungsten to "green" neodymium: A century of critical metals at a glance", ''Ore Geology Reviews'', vol. 64, January, pp. 455–458, . * Ephraim Chambers, Chambers E. 1743,
Metal
, in ''Cyclopedia: Or an Universal Dictionary of Arts and Sciences (etc.)'', vol. 2, D. Midwinter, London. * Chandler D. E. & Roberson R. W. 2009, ''Bioimaging: Current Concepts in Light & Electron Microscopy'', Jones & Bartlett Publishers, Boston, . * Chawla N. & Chawla K. K. 2013, ''Metal matrix composites'', 2nd ed., Springer Science+Business Media, New York, . * Chen J. & Huang K. 2006, "A new technique for extraction of platinum group metals by pressure cyanidation", ''Hydrometallurgy'', vol. 82, nos. 3–4, pp. 164–171, . * Matthew Choptuik, Choptuik M. W., Lehner L. & Pretorias F. 2015, "Probing strong-field gravity through numerical simulation", in Abhay Ashtekar, A. Ashtekar, Beverly Berger, B. K. Berger, J. Isenberg & M. MacCallum (eds), ''General Relativity and Gravitation: A Centennial Perspective'', Cambridge University Press, Cambridge, . * Brian Clegg (writer), Clegg B 2014,
Osmium tetroxide
, ''Chemistry World'', accessed 2 September 2016. * Close F. 2015, ''Nuclear Physics: A Very Short Introduction'', Oxford University Press, Oxford, . * Clugston M & Flemming R 2000, ''Advanced Chemistry'', Oxford University, Oxford, . * Cole M., Lindeque P., Halsband C. & Galloway T. S. 2011, "Microplastics as contaminants in the marine environment: A review", ''Marine Pollution Bulletin'', vol. 62, no. 12, pp. 2588–2597, . * Cole S. E. & Stuart K. R. 2000, "Nuclear and cortical histology for bright-field microscopy, brightfield microscopy", in D. J. Asai & J. D. Forney (eds), ''Methods in Cell Biology'', vol. 62, Academic Press, San Diego, pp. 313–322, . * Cotton S. A. 1997, ''Chemistry of Precious Metals'', Blackie Academic & Professional, London, . * Cotton S. 2006, ''Lanthanide and Actinide Chemistry'', reprinted with corrections 2007, John Wiley & Sons, Chichester, . * Cox P. A. 1997, ''The elements: Their Origin, Abundance and Distribution'', Oxford University Press, Oxford, . * Crundwell F. K., Moats M. S., Ramachandran V., Robinson T. G. & Davenport W. G. 2011, ''Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals'', Elsevier, Kidlington, Oxford, . * Cui X-Y., Li S-W., Zhang S-J., Fan Y-Y., Ma L. Q. 2015, "Toxic metals in children's toys and jewelry: Coupling bioaccessibility with risk assessment", ''Environmental Pollution (journal), Environmental Pollution'', vol. 200, pp. 77–84, . * Dapena J. & Teves M. A. 1982, "Influence of the diameter of the hammer head on the distance of a hammer throw", ''Research Quarterly for Exercise and Sport'', vol. 53, no. 1, pp. 78–81, . * De Zuane J. 1997, ''Handbook of Drinking Water Quality,'' 2nd ed., John Wiley & Sons, New York, . * United States Department of the Navy, Department of the Navy 2009,
Gulf of Alaska Navy Training Activities: Draft Environmental Impact Statement/Overseas Environmental Impact Statement
', U.S. Government, accessed 21 August 2016. * Deschlag J. O. 2011, "Nuclear fission", in A. Vértes, S. Nagy, Z. Klencsár, R. G. Lovas, F. Rösch (eds), ''Handbook of Nuclear Chemistry'', 2nd ed., Springer Science+Business Media, Dordrecht, pp. 223–280, . * Desoize B. 2004, "Metals and metal compounds in cancer treatment", ''Anticancer Research'', vol. 24, no. 3a, pp. 1529–1544, . * Dev N. 2008, 'Modelling Selenium Fate and Transport in Great Salt Lake Wetlands', PhD dissertation, University of Utah, ProQuest, Ann Arbor, Michigan, . * Vincent Di Maio, Di Maio V. J. M. 2001, ''Forensic Pathology,'' 2nd ed., CRC Press, Boca Raton, . * Vincent Di Maio, Di Maio V. J. M. 2016, ''Gunshot Wounds: Practical Aspects of Firearms, Ballistics, and Forensic Techniques'', 3rd ed., CRC Press, Boca Raton, Florida, .
Duffus J. H.
2002,
'Heavy metals'—A meaningless term?"
''Pure and Applied Chemistry'', vol. 74, no. 5, pp. 793–807, . * Dunn P. 2009
''Unusual metals could forge new cancer drugs''
University of Warwick, accessed 23 March 2016. * Ebbing D. D. & Gammon S. D. 2017, ''General Chemistry'', 11th ed., Cengage Learning, Boston, . * Edelstein N. M., Fuger J., Katz J. L. & Morss L. R. 2010, "Summary and comparison of properties of the actinde and transactinide elements," in L. R. Morss, N. M. Edelstein & J. Fuger (eds), ''The Chemistry of the Actinide and Transactinide Elements'', 4th ed., vol. 1–6, Springer Publishing, Springer, Dordrecht, pp. 1753–1835, . * Eisler R. 1993,
Zinc Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review
', Biological Report 10, United States Department of the Interior, U. S. Department of the Interior, Laurel, Maryland, accessed 2 September 2016. * Elliott S. B. 1946, ''The Alkaline-earth and Heavy-metal Soaps, '' Reinhold Publishing Corporation, New York. * John Emsley, Emsley J. 2011,
Nature's Building Blocks
', new edition, Oxford University Press, Oxford, . * Everts S. 2016,
, ''Chemical & Engineering News'', vol. 94, no. 33, pp. 24–26. * Fournier J. 1976, "Bonding and the electronic structure of the actinide metals," ''Journal of Physics and Chemistry of Solids'', vol 37, no. 2, pp. 235–244, . * Frick J. P. (ed.) 2000, ''Woldman's Engineering Alloys'', 9th ed., ASM International (society), ASM International, Materials Park, Ohio, . * Frommer H. H. & Stabulas-Savage J. J. 2014, ''Radiology for the Dental Professional'', 9th ed., Mosby (imprint), Mosby Inc., St. Louis, Missouri, . *Gidding J. C. 1973, ''Chemistry, Man, and Environmental Change: An Integrated Approach'', Canfield Press, New York, . * Leopold Gmelin, Gmelin L. 1849, ''Hand-book of chemistry'', vol. III, Metals, translated from the German by H. Watts, Cavendish Society, London. * Goldsmith R. H. 1982, "Metalloids", ''Journal of Chemical Education'', vol. 59, no. 6, pp. 526–527, . * Gorbachev V. M., Zamyatnin Y. S. & Lbov A. A. 1980, ''Nuclear Reactions in Heavy Elements: A Data Handbook,'' Pergamon Press, Oxford, . * Gordon Gordh, Gordh G. & Headrick D. 2003, ''A Dictionary of Entomology'', CABI Publishing, Wallingford, . * Greenberg B. R. & Patterson D. 2008, ''Art in Chemistry; Chemistry in Art'', 2nd ed., Teachers Ideas Press, Westport, Connecticut, . * Gribbon J. 2016, ''13.8: The Quest to Find the True Age of the Universe and the Theory of Everything'', Yale University Press, New Haven, . * Gschneidner Jr., K. A. 1975, ''Inorganic compounds'', in C. T. Horowitz (ed.), ''Scandium: Its Occurrence, Chemistry, Physics, Metallurgy, Biology and Technology'', Academic Press, London, pp. 152–251, . * Guandalini G. S., Zhang L., Fornero E., Centeno J. A., Mokashi V. P., Ortiz P. A., Stockelman M. D., Osterburg A. R. & Chapman G. G. 2011, "Tissue distribution of tungsten in mice following oral exposure to sodium tungstate," ''Chemical Research in Toxicology'', vol. 24, no. 4, pp 488–493, . * Guney M. & Zagury G. J. 2012, "Heavy metals in toys and low-cost jewelry: Critical review of U.S. and Canadian legislations and recommendations for testing", ''Environmental Science & Technology'', vol. 48, pp. 1238–1246, . * Habashi F. 2009,
Gmelin and his Handbuch"
''Bulletin for the History of Chemistry'', vol. 34, no. 1, pp. 30–1. * Hadhazy A. 2016,
Galactic 'gold mine' explains the origin of nature's heaviest elements
, ''Science Spotlights'', 10 May 2016, accessed 11 July 2016. * William Kenneth Hartmann, Hartmann W. K. 2005, ''Moons & Planets'', 5th ed., Thomson Brooks/Cole, Belmont, California, . * Harvey P. J., Handley H. K. & Taylor M. P. 2015, "Identification of the sources of metal (lead) contamination in drinking waters in north-eastern Tasmania using lead isotopic compositions," ''Environmental Science and Pollution Research'', vol. 22, no. 16, pp. 12276–12288, . * Hasan S. E. 1996, ''Geology and Hazardous Waste Management'', Prentice Hall, Upper Saddle River, New Jersey, . * Hawkes S. J. 1997, "What is a "heavy metal"?", ''Journal of Chemical Education'', vol. 74, no. 11, p. 1374, . * Haynes W. M. 2015, ''CRC Handbook of Chemistry and Physics'', 96th ed., CRC Press, Boca Raton, Florida, . * Hendrickson D. J. 2916, "Effects of early experience on brain and body", in D. Alicata, N. N. Jacobs, A. Guerrero and M. Piasecki (eds), ''Problem-based Behavioural Science and Psychiatry'' 2nd ed., Springer, Cham, pp. 33–54, . * Hermann A., Roald Hoffmann, Hoffmann R. & Neil Ashcroft, Ashcroft N. W. 2013,
Condensed astatine: Monatomic and metallic
, ''Physical Review Letters'', vol. 111, pp. 11604–1−11604-5, . * Herron N. 2000, "Cadmium compounds," in ''Kirk-Othmer Encyclopedia of Chemical Technology'', vol. 4, John Wiley & Sons, New York, pp. 507–523, . * Hoffman D. C., Lee D. M. & Pershina V. 2011, "Transactinide elements and future elements," in L. R. Morss, N. Edelstein, J. Fuger & J. J. Katz (eds), ''The Chemistry of the Actinide and Transactinide Elements'', 4th ed., vol. 3, Springer, Dordrecht, pp. 1652–1752, . * Hofmann S. 2002, ''On Beyond Uranium: Journey to the End of the Periodic Table'', Taylor & Francis, London, . * Housecroft J. E. 2008, ''Inorganic Chemistry'', Elsevier, Burlington, Massachusetts, . * Howell N., Lavers J., Paterson D., Garrett R. & Banati R. 2012,
Trace metal distribution in feathers from migratory, pelagic birds
', Australian Nuclear Science and Technology Organisation, accessed 3 May 2014. * Hübner R., Astin K. B. & Herbert R. J. H. 2010, " 'Heavy metal'—time to move on from semantics to pragmatics?", ''Journal of Environmental Monitoring'', vol. 12, pp. 1511–1514, . * Ikehata K., Jin Y., Maleky N. & Lin A. 2015, "Heavy metal pollution in water resources in China—Occurrence and public health implications", in S. K. Sharma (ed.), ''Heavy Metals in Water: Presence, Removal and Safety,'' Royal Society of Chemistry, Cambridge, pp. 141–167, . * International Antimony Association 2016,
Antimony compounds
', accessed 2 September 2016. * International Platinum Group Metals Association n.d.,
The Primary Production of Platinum Group Metals (PGMs)
', accessed 4 September 2016. * Ismail A. F., Khulbe K. & Matsuura T. 2015, ''Gas Separation Membranes: Polymeric and Inorganic'', Springer, Cham, Switzerland, . * IUPAC 2016,
IUPAC is naming the four new elements nihonium, moscovium, tennessine, and oganesson
accessed 27 August 2016. * Iyengar G. V. 1998, "Reevaluation of the trace element content in Reference Man", ''Radiation Physics and Chemistry,'' vol. 51, nos 4–6, pp. 545–560, * Jackson J. & Summitt J. 2006, ''The Modern Guide to Golf Clubmaking: The Principles and Techniques of Component Golf Club Assembly and Alteration'', 5th ed., Hireko Trading Company, City of Industry, California, . * Järup L 2003, "Hazards of heavy metal contamination", ''British Medical Bulletin'', vol. 68, no. 1, pp. 167–182, . * Jones C. J. 2001, ''d- and f-Block Chemistry'', Royal Society of Chemistry, Cambridge, . * Kantra S. 2001, "What's new", ''Popular Science'', vol. 254, no. 4, April, p. 10. * Keller C., Wolf W. & Shani J. 2012, "Radionuclides, 2. Radioactive elements and artificial radionuclides", in Fritz Ullmann, F. Ullmann (ed.), ''Ullmann's Encyclopedia of Industrial Chemistry'', vol. 31, Wiley-VCH, Weinheim, pp. 89–117, . * King R. B. 1995, ''Inorganic Chemistry of Main Group Elements'', Wiley-VCH, New York, . * Izaak Kolthoff, Kolthoff I. M. & Elving P. J. FR 1964, ''Treatise on Analytical Chemistry'', part II, vol. 6, Interscience Encyclopedia, New York, . * Korenman I. M. 1959, "Regularities in properties of thallium", ''Journal of General Chemistry of the USSR'', English translation, Consultants Bureau, New York, vol. 29, no. 2, pp. 1366–90, . * Kozin L. F. & Hansen S. C. 2013, ''Mercury Handbook: Chemistry, Applications and Environmental Impact'', RSC Publishing, Cambridge, . * Kumar R., Srivastava P. K., Srivastava S. P. 1994, "Leaching of heavy metals (Cr, Fe, and Ni) from stainless steel utensils in food simulates and food materials", ''Bulletin of Environmental Contamination and Toxicology'', vol. 53, no. 2, , pp. 259–266. * Lach K., Steer B., Gorbunov B., Mička V. & Muir R. B. 2015, "Evaluation of exposure to airborne heavy metals at gun shooting ranges", ''The Annals of Occupational Hygiene'', vol. 59, no. 3, pp. 307–323, . * Landis W., Sofield R. & Yu M-H. 2010, ''Introduction to Environmental Toxicology: Molecular Substructures to Ecological Landscapes'', 4th ed., CRC Press, Boca Raton, Florida, . * Lane T. W., Saito M. A., George G. N., Pickering, I. J., Prince R. C. & Morel F. M. M. 2005, "Biochemistry: A cadmium enzyme from a marine diatom", ''Nature (journal), Nature'', vol. 435, no. 7038, p. 42, . * Lee J. D. 1996, ''Concise Inorganic Chemistry,'' 5th ed., Blackwell Science, Oxford, . * Leeper G. W. 1978, ''Managing the Heavy Metals on the Land'' Marcel Dekker, New York, . * Lemly A. D. 1997, "A teratogenic deformity index for evaluating impacts of selenium on fish populations", ''Ecotoxicology and Environmental Safety'', vol. 37, no. 3, pp. 259–266, . * Lide D. R. (ed.) 2004, ''CRC Handbook of Chemistry and Physics'', 85th ed., CRC Press, Boca Raton, Florida, . * Liens J. 2010, "Heavy metals as pollutants", in B. Warf (ed.), ''Encyclopaedia of Geography'', Sage Publications, Thousand Oaks, California, pp. 1415–1418, . * Lima E., Guerra R., Lara V. & Guzmán A. 2013, "Gold nanoparticles as efficient antimicrobial agents for ''Escherichia coli'' and ''Salmonella typhi'' " ''Chemistry Central'', vol. 7:11, . * Litasov K. D. & Shatskiy A. F. 2016, "Composition of the Earth's core: A review", ''Russian Geology and Geophysics'', vol. 57, no. 1, pp. 22–46, . * Livesey A. 2012, ''Advanced Motorsport Engineering'', Routledge, London, . * Livingston R. A. 1991, "Influence of the Environment on the Patina of the Statue of Liberty", ''Environmental Science & Technology,'' vol. 25, no. 8, pp. 1400–1408, . * Longo F. R. 1974, ''General Chemistry: Interaction of Matter, Energy, and Man'', McGraw-Hill, New York, . * Love M. 1998, ''Phasing Out Lead from Gasoline: Worldwide Experience and Policy Implications,'' World Bank Technical Paper volume 397, The World Bank, Washington DC, . * Lyman W. J. 1995, "Transport and transformation processes", in ''Fundamentals of Aquatic Toxicology'', G. M. Rand (ed.), Taylor & Francis, London, pp. 449–492, . * Macintyre J. E. 1994, ''Dictionary of inorganic compounds'', supplement 2, Dictionary of Inorganic Compounds, vol. 7, Chapman & Hall, London, . * MacKay K. M., MacKay R. A. & Henderson W. 2002, ''Introduction to Modern Inorganic Chemistry'', 6th ed., Nelson Thornes, Cheltenham, . * Magee R. J. 1969, ''Steps to Atomic Power'', Cheshire for La Trobe University, Melbourne. * Magill F. N. I (ed.) 1992, ''Magill's Survey of Science'', Physical Science series, vol. 3, Salem Press, Pasadena, . * Martin M. H. & Coughtrey P. J. 1982, ''Biological Monitoring of Heavy Metal Pollution'', Applied Science Publishers, London, . * Massarani M. 2015,
Brazilian mine disaster releases dangerous metals
" ''Chemistry World'', November 2015, accessed 16 April 2016. * Masters C. 1981, ''Homogenous Transition-metal Catalysis: A Gentle Art'', Chapman and Hall, London, . * Matyi R. J. & Baboian R. 1986, "An X-ray Diffraction Analysis of the Patina of the Statue of Liberty", ''Powder Diffraction,'' vol. 1, no. 4, pp. 299–304, . * McColm I. J. 1994, ''Dictionary of Ceramic Science and Engineering'', 2nd ed., Springer Science+Business Media, New York, . * McCurdy R. M. 1975, ''Qualities and quantities: Preparation for College Chemistry'', Harcourt Brace Jovanovich, New York, . * McLemore V. T. (ed.) 2008, ''Basics of Metal Mining Influenced Water'', vol. 1, Society for Mining, Metallurgy, and Exploration, Littleton, Colorado, . * McQueen K. G. 2009, ''Regolith geochemistry'', in K. M. Scott & C. F. Pain (eds), ''Regolith Science'', CSIRO Publishing, Collingwood, Victoria, . * Joseph William Mellor, Mellor J. W. 1924, ''A comprehensive Treatise on Inorganic and Theoretical Chemistry'', vol. 5, Longmans, Green and Company, London. * Moore J. W. & Ramamoorthy S. 1984, ''Heavy Metals in Natural Waters: Applied Monitoring and Impact Assessment'', Springer Verlag, New York, . * Morris C. G. 1992, ''Academic Press Dictionary of Science and Technology'', Harcourt Brace Jovanovich, San Diego, . * Morstein J. H. 2005, "Fat Man", in E. A. Croddy & Y. Y. Wirtz (eds), ''Weapons of Mass Destruction: An Encyclopedia of Worldwide Policy, Technology, and History'', ABC-CLIO, Santa Barbara, California, . * Moselle B. (ed.) 2005, ''2004 National Home Improvement Estimator'', Craftsman Book Company, Carlsbad, California, . * Naja G. M. & Volesky B. 2009, "Toxicity and sources of Pb, Cd, Hg, Cr, As, and radionuclides", in L. K. Wang, J. P. Chen, Y. Hung & N. K. Shammas, ''Heavy Metals in the Environment'', CRC Press, Boca Raton, Florida, . * Nakbanpote W., Meesungneon O. & Prasad M. N. V. 2016, "Potential of ornamental plants for phytoremediation of heavy metals and income generation", in M. N. V. Prasad (ed.), ''Bioremediation and Bioeconomy'', Elsevier, Amsterdam, pp. 179–218, . * Nathans M. W. 1963, ''Elementary Chemistry'', Prentice Hall, Englewood Cliffs, New Jersey. * National Materials Advisory Board 1971, ''Trends in the Use of Depleted Uranium'', National Academy of Sciences – National Academy of Engineering, Washington DC. * National Materials Advisory Board 1973, ''Trends in Usage of Tungsten'', National Academy of Sciences – National Academy of Engineering, Washington DC. * National Organization for Rare Disorders 2015,
Heavy metal poisoning
', accessed 3 March 2016. * Natural Resources Canada 2015,
Generation of the Earth's magnetic field
, accessed 30 August 2016. * Nieboer E. & Richardson D. 1978, "Lichens and 'heavy metals' ", ''International Lichenology Newsletter'', vol. 11, no. 1, pp. 1–3. * Nieboer E. & Richardson D. H. S. 1980, "The replacement of the nondescript term 'heavy metals' by a biologically and chemically significant classification of metal ions", ''Environmental Pollution (journal), Environmental Pollution Series B, Chemical and Physical'', vol. 1, no. 1, pp. 3–26, . * Nzierżanowski K. & Gawroński S. W. 2012,
Heavy metal concentration in plants growing on the vicinity of railroad tracks: a pilot study
, ''Challenges of Modern Technology'', vol. 3, no. 1, pp. 42–45, , accessed 21 August 2016. * Ohlendorf H. M. 2003, "Ecotoxicology of selenium", in D. J. Hoffman, B. A. Rattner, G. A. Burton & John Cairns (biochemist), J. Cairns, ''Handbook of Ecotoxicology'', 2nd ed., Lewis Publishers, Boca Raton, pp. 466–491, . * Ondreička R., Kortus J. & Ginter E. 1971, "Aluminium, its absorption, distribution, and effects on phosphorus metabolism", in S. C. Skoryna & D. Waldron-Edward (eds), ''Intestinal Absorption of Metal Ions, Trace Elements and Radionuclides'', Pergamon press, Oxford. * Ong K. L., Tan T. H. & Cheung W. L. 1997, "Potassium permanganate poisoning—a rare cause of fatal poisoning", ''Journal of Accident & Emergency Medicine'', vol. 14, no. 1, pp. 43–45, . * ''Oxford English Dictionary'' 1989, 2nd ed., Oxford University Press, Oxford, . * Pacheco-Torgal F., Jalali S. & Fucic A. (eds) 2012, ''Toxicity of building materials'', Woodhead Publishing, Oxford, . * Thanu Padmanabhan, Padmanabhan T. 2001, ''Theoretical Astrophysics'', vol. 2, Stars and Stellar Systems, Cambridge University Press, Cambridge, . * Pan W. & Dai J. 2015, "ADS based on linear accelerators", in W. Chao & W. Chou (eds), ''Reviews of accelerator science and technology'', vol. 8, Accelerator Applications in Energy and Security, World Scientific, Singapore, pp. 55–76, . * Parish R. V. 1977, ''The Metallic Elements'', Longman, New York, . * Perry J. & Vanderklein E. L. ''Water Quality: Management of a Natural Resource,'' Blackwell Science, Cambridge, Massachusetts . * Pickering N. C. 1991, ''The Bowed String: Observations on the Design, Manufacture, Testing and Performance of Strings for Violins, Violas and Cellos'', Amereon, Mattituck, New York. * Podosek F. A. 2011, "Noble gases", in H. D. Holland & Karl Turekian, K. K. Turekian (eds), ''Isotope Geochemistry: From the Treatise on Geochemistry'', Elsevier, Amsterdam, pp. 467–492, . * Podsiki C. 2008,
Heavy metals, their salts, and other compounds
, ''American Institute for Conservation, AIC News,'' November, special insert, pp. 1–4. * Preschel J. July 29, 2005,
Green bullets not so eco-friendly
, ''CBS News'', accessed 18 March 2016. * Preuss P. 17 July 2011,
What keeps the Earth cooking?
" Berkeley Lab, accessed 17 July 2016. * Carlos Prieto (cellist), Prieto C. 2011, ''The Adventures of a Cello: Revised Edition, with a New Epilogue,'' University of Texas Press, Austin, * Raghuram P., Soma Raju I. V. & Sriramulu J. 2010, "Heavy metals testing in active pharmaceutical ingredients: an alternate approach", ''Pharmazie'', vol. 65, no. 1, pp. 15–18, . * Rainbow P. S. 1991, "The biology of heavy metals in the sea", in J. Rose (ed.), ''Water and the Environment'', Gordon and Breach Science Publishers, Philadelphia, pp. 415–432, . * Rand G. M., Wells P. G. & McCarty L. S. 1995, "Introduction to aquatic toxicology", in G. M. Rand (ed.), ''Fundamentals of Aquatic Toxicology: Effects, Environmental Fate and Risk Assessment'', 2nd ed., Taylor & Francis, London, pp. 3–70, . * Rankin W. J. 2011, ''Minerals, Metals and Sustainability: Meeting Future Material Needs'', CSIRO Publishing, Collingwood, Victoria, . * Rasic-Milutinovic Z. & Jovanovic D. 2013, "Toxic metals", in M. Ferrante, G. Oliveri Conti, Z. Rasic-Milutinovic & D. Jovanovic (eds), ''Health Effects of Metals and Related Substances in Drinking Water'', International Water Association, IWA Publishing, London, . * Raymond R. 1984, ''Out of the Fiery Furnace: The Impact of Metals on the History of Mankind'', Macmillan Publishing, Macmillan, South Melbourne, . * Rebhandl W., Milassin A., Brunner L., Steffan I., Benkö T., Hörmann M., Burschen J. 2007, "In vitro study of ingested coins: Leave them or retrieve them?", ''Journal of Paediatric Surgery'', vol. 42, no. 10, pp. 1729–1734, . * Rehder D. 2010, ''Chemistry in Space: From Interstellar Matter to the Origin of Life'', Wiley-VCH, Weinheim, . * Renner H., Schlamp G., Kleinwächter I., Drost E., Lüchow H. M., Tews P., Panster P., Diehl M., Lang J., Kreuzer T., Knödler A., Starz K. A., Dermann K., Rothaut J., Drieselmann R., Peter C. & Schiele R. 2012, "Platinum Group Metals and compounds", in F. Ullmann (ed.), ''Ullmann's Encyclopedia of Industrial Chemistry'', vol. 28, Wiley-VCH, Weinheim, pp. 317–388, . * Reyes J. W. 2007,
Environmental Policy as Social Policy? The Impact of Childhood Lead Exposure on Crime
', National Bureau of Economic Research Working Paper 13097, accessed 16 October 2016. * Ian Ridpath, Ridpath I. (ed.) 2012, ''Oxford Dictionary of Astronomy'', 2nd ed. rev., Oxford University Press, New York, . * Rockhoff H. 2012, ''America's Economic Way of War: War and the US Economy from the Spanish–American War to the Persian Gulf War'', Cambridge University Press, Cambridge, . * Roe J. & Roe M. 1992, "World's coinage uses 24 chemical elements", ''World Coinage News'', vol. 19, no. 4, pp. 24–25; no. 5, pp. 18–19. * Russell A. M. & Lee K. L. 2005, ''Structure–Property Relations in Nonferrous Metals'', John Wiley & Sons, Hoboken, New Jersey, . * Rusyniak D. E., Arroyo A., Acciani J., Froberg B., Kao L. & Furbee B. 2010, "Heavy metal poisoning: Management of intoxication and antidotes", in A. Luch (ed.), ''Molecular, Clinical and Environmental Toxicology'', vol. 2, Birkhäuser Verlag, Basel, pp. 365–396, . * Ryan J. 2012, ''Personal Financial Literacy'', 2nd ed., South-Western, Mason, Ohio, . * Samsonov G. V. (ed.) 1968, ''Handbook of the Physicochemical Properties of the Elements'', IFI-Plenum, New York, . * Sanders R. 2003,
Radioactive potassium may be major heat source in Earth's core
" ''UCBerkelyNews'', 10 December, accessed 17 July 20016. * Schweitzer P. A. 2003, ''Metallic materials: Physical, Mechanical, and Corrosion properties'', Marcel Dekker, New York, . * George K. Schweitzer, Schweitzer G. K. & Pesterfield L. L. 2010, ''The Aqueous Chemistry of the Elements'', Oxford University Press, Oxford, . * Scott R. M. 1989, ''Chemical Hazards in the Workplace'', CRC Press, Boca Raton, Orlando, . * Scoullos M. (ed.), Vonkeman G. H., Thornton I. & Makuch Z. 2001, ''Mercury — Cadmium — Lead Handbook for Sustainable Heavy Metals Policy and Regulation'', Kluwer Academic Publishers, Dordrecht, . * Selinger B. 1978, ''Chemistry in the Market Place'', 2nd ed., Australian National University Press, Canberra, . * Seymour R. J. & O'Farrelly J. 2012, "Platinum Group Metals", ''Kirk-Other Encyclopaedia of Chemical Technology'', John Wiley & Sons, New York, . * Shaw B. P., Sahu S. K. & Mishra R. K. 1999, "Heavy metal induced oxidative damage in terrestrial plants", in M. N. V. Prased (ed.), ''Heavy Metal Stress in Plants: From Biomolecules to Ecosystems'' Springer-Verlag, Berlin, . * Shedd K. B. 2002,
Tungsten"
''Minerals Yearbook'', United States Geological Survey. * Nevil Sidgwick, Sidgwick N. V. 1950, ''The Chemical Elements and their Compounds'', vol. 1, Oxford University Press, London. * Silva R. J. 2010, "Fermium, mendelevium, nobelium, and lawrencium", in L. R. Morss, N. Edelstein & J. Fuger (eds), ''The Chemistry of the Actinide and Transactinide Elements'', vol. 3, 4th ed., Springer, Dordrecht, pp. 1621–1651, . * Spolek G. 2007, "Design and materials in fly fishing", in A. Subic (ed.), ''Materials in Sports Equipment'', Volume 2, Woodhead Publishing, Abington, Cambridge, pp. 225–247, . * Stankovic S. & Stankocic A. R. 2013, "Bioindicators of toxic metals", in E. Lichtfouse, J. Schwarzbauer, D. Robert 2013, ''Green materials for energy, products and depollution'', Springer, Dordrecht, , pp. 151–228. * State Water Control Resources Board 1987, ''Toxic substances monitoring program'', issue 79, part 20 of the Water Quality Monitoring Report, Sacramento, California. * Technical Publications 1953, ''Fire Engineering'', vol. 111, p. 235, . * The Minerals, Metals and Materials Society,
Light Metals Division 2016
', accessed 22 June 2016. * ''The
United States Pharmacopeia
The ''United States Pharmacopeia'' (''USP'') is a pharmacopeia (compendium of drug information) for the United States published annually by the United States Pharmacopeial Convention (usually also called the USP), a nonprofit organization that ...
'' 1985, 21st revision, The United States Pharmacopeial Convention, Rockville, Maryland, .
* Thorne P. C. L. & Roberts E. R. 1943, ''Fritz Ephraim Inorganic Chemistry'', 4th ed., Gurney and Jackson, London.
* Tisza M. 2001, ''Physical Metallurgy for Engineers'', ASM International, Materials Park, Ohio, .
* Tokar E. J., Boyd W. A., Freedman J. H. & Wales M. P. 2013,Toxic effects of metals
, in C. D. Klaassen (ed.), ''Casarett and Doull's Toxicology: the Basic Science of Poisons'', 8th ed., McGraw-Hill Medical, New York, , accessed 9 September 2016 . * Tomasik P. & Ratajewicz Z. 1985, ''Pyridine metal complexes,'' vol. 14, no. 6A, The Chemistry of Heterocyclic Compounds, John Wiley & Sons, New York, . * Topp N. E. 1965, ''The Chemistry of the Rare-earth Elements'', Elsevier Publishing Company, Amsterdam. * Torrice M. 2016,
How lead ended up in Flint's tap water
" ''Chemical & Engineering News'', vol. 94, no. 7, pp. 26–27. * Tretkoff E. 2006,
March 20, 1800: Volta describes the Electric Battery
, ''APS News, This Month in Physics History'', American Physical Society, accessed 26 August 2016. * Uden P. C. 2005, 'Speciation of Selenium,' in R. Cornelis, J. Caruso, H. Crews & K. Heumann (eds), ''Handbook of Elemental Speciation II: Species in the Environment, Food, Medicine and Occupational Health,'' John Wiley & Sons, Chichester, pp. 346–65, . * United States Environmental Protection Agency 1988, ''Ambient Aquatic Life Water Quality Criteria for Antimony (III),'' draft, Office of Research and Development, Environmental Research Laboratories, Washington. * United States Environmental Protection Agency 2014,
Technical Fact Sheet–Tungsten
', accessed 27 March 2016. * United States Government 2014,
Toxic Pollutant List
', Code of Federal Regulations, 40 CFR 401.15., accessed 27 March 2016. * Valkovic V. 1990, "Origin of trace element requirements by living matter", in B. Gruber & J. H. Yopp (eds), ''Symmetries in Science IV: Biological and biophysical systems'', Plenum Press, New York, pp. 213–242, . * VanGelder K. T. 2014, ''Fundamentals of Automotive Technology: Principles and Practice'', Jones & Bartlett Learning, Burlington MA, . * Venner M., Lessening M., Pankani D. & Strecker E. 2004,
Identification of Research Needs Related to Highway Runoff Management
', Transportation Research Board, Washington DC, , accessed 21 August 2016. * Venugopal B. & Luckey T. D. 1978, ''Metal Toxicity in Mammals'', vol. 2, Plenum Press, New York, . * Vernon R. E. 2013, "Which elements are metalloids", ''Journal of Chemical Education'', vol. 90, no. 12, pp. 1703–1707, . * Volesky B. 1990, ''Biosorption of Heavy Metals'', CRC Press, Boca Raton, . * von Gleich A. 2013, "Outlines of a sustainable metals industry", in A. von Gleich, R. U. Ayres & S. Gößling-Reisemann (eds), ''Sustainable Metals Management'', Springer, Dordrecht, pp. 3–40, . * von Zeerleder A. 1949, ''Technology of Light Metals'', Elsevier Publishing Company, New York. * Warth A. H. 1956, ''The Chemistry and Technology of Waxes'', Reinhold Publishing Corporation, New York. * Spencer R. Weart, Weart S. R. 1983, "The discovery of nuclear fission and a nuclear physics paradigm", in W. Shea (ed.), ''Otto Hahn and the Rise of Nuclear Physics'', D. Reidel Publishing Company, Dordrecht, pp. 91–133, . * Weber D. J. & Rutula W. A. 2001, "Use of metals as microbicides in preventing infections in healthcare", in ''Disinfection, Sterilization, and Preservation'', 5th ed., S. S. Block (ed.), Lippincott, Williams & Wilkins, Philadelphia, . * Welter G. 1976, ''Cleaning and Preservation of Coins and Medals'', S. J. Durst, New York, . * White C. 2010, ''Projectile Dynamics in Sport: Principles and Applications'', Routledge, London, . * Wiberg N. 2001, ''Inorganic Chemistry'', Academic Press, San Diego, . * Wijayawardena M. A. A., Megharaj M. & Naidu R. 2016, "Exposure, toxicity, health impacts and bioavailability of heavy metal mixtures", in D. L. Sparks, ''Advances in Agronomy'', vol. 138, pp. 175–234, Academic Press, London, . * Wingerson L. 1986,
America cleans up Liberty
, ''New Scientist,'' 25 December/1 January 1987, pp. 31–35, accessed 1 October 2016. * Wong M. Y., Hedley G. J., Xie G., Kölln L. S, Samuel I. D. W., Pertegaś A., Bolink H. J., Mosman-Colman, E., "Light-emitting electrochemical cells and solution-processed organic light-emitting diodes using small molecule organic thermally activated delayed fluorescence emitters", ''Chemistry of Materials'', vol. 27, no. 19, pp. 6535–6542, . * Wulfsberg G. 1987, ''Principles of Descriptive Inorganic Chemistry'', Cengage Learning, Brooks/Cole Publishing Company, Monterey, California, . * Wulfsberg G. 2000, ''Inorganic Chemistry'', University Science Books, Sausalito, California, . * Yadav J. S., Antony A., Subba Reddy, B. V. 2012, "Bismuth(III) salts as synthetic tools in organic transformations", in T. Ollevier (ed.), ''Bismuth-mediated Organic Reactions'', Topics in Current Chemistry 311, Springer, Heidelberg, . * Yang D. J., Jolly W. L. & O'Keefe A. 1977, "Conversion of hydrous germanium(II) oxide to germynyl sesquioxide, (HGe)2O3", ''Inorganic Chemistry (journal), 'Inorganic Chemistry'', vol. 16, no. 11, pp. 2980–2982, . * Yousif N. 2007, ''Geochemistry of stream sediment from the state of Colorado using NURE data'', ETD Collection for the University of Texas, El Paso
paper AAI3273991
Further reading
Definition and usage * Ali H. & Khan E. 2017, "What are heavy metals? Long-standing controversy over the scientific use of the term 'heavy metals'—proposal of a comprehensive definition", ''Toxicological & Environmental Chemistry,'' pp. 1–25, . Suggests defining heavy metals as "naturally occurring metals having atomic number (Z) greater than 20 and an elemental density greater than 5 g cm−3".2002,
Heavy metals'—A meaningless term?"
''Pure and Applied Chemistry'', vol. 74, no. 5, pp. 793–807, . Includes a survey of the term's various meanings. * Hawkes S. J. 1997,
What is a 'heavy metal'?
, ''Journal of Chemical Education'', vol. 74, no. 11, p. 1374, . A chemist's perspective. * Hübner R., Astin K. B. & Herbert R. J. H. 2010, Heavy metal'—time to move on from semantics to pragmatics?", ''Journal of Environmental Monitoring'', vol. 12, pp. 1511–1514, . Finds that, despite its lack of specificity, the term appears to have become part of the language of science. Toxicity and biological role * Baird C. & Cann M. 2012, ''Environmental Chemistry'', 5th ed., chapter 12, "Toxic heavy metals", W. H. Freeman and Company, New York, . Discusses the use, toxicity, and distribution of Hg, Pb, Cd, As, and Cr. * Nieboer E. & Richardson D. H. S. 1980, "The replacement of the nondescript term 'heavy metals' by a biologically and chemically significant classification of metal ions", ''Environmental Pollution Series B, Chemical and Physical'', vol. 1, no. 1, pp. 3–26, . A widely cited paper, focusing on the biological role of heavy metals. Formation * Hadhazy A. 2016,
Galactic 'gold mine' explains the origin of nature's heaviest elements
, ''Science Spotlights'', 10 May, accessed 11 July 2016 Uses * Koehler C. S. W. 2001,
, ''Chemistry Chronicles'', American Chemical Society, accessed 11 July 2016 * Morowitz N. 2006, "The heavy metals", ''Modern Marvels'', season 12, episode 14, History (U.S. TV channel), HistoryChannel.com * Öhrström L. 2014,
Tantalum oxide
, ''Chemistry World'', 24 September, accessed 4 October 2016. The author explains how tantalum(V) oxide banished brick-sized mobile phones. Also available as
podcast
External links
* {{Authority control Metals Metallic elements Sets of chemical elements