|
Organotin
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide (), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory. Structure Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful. Organic derivatives of tin(IV) The tetraorgano derivatives are invariably tetrahedral. Compounds of the type SnRR'R''R have been resolved into individual enantiomers. Organotin halides Organotin chlorides have the formula for values of ''n'' up to 3. Bromides, iodides, and fluorides are also known but less important. These ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethyltin Chloride
Trimethyltin chloride is an organotin compound with the formula . It is a white solid that is highly toxic and malodorous. It is susceptible to hydrolysis. Synthesis Trimethyltin chloride can be prepared by the Redistribution (chemistry), redistribution reaction of tetramethyltin with tin tetrachloride. : This is the Kocheshkov redistribution reaction. It is performed under an inert atmosphere, such as argon, typically with no solvent. A second route to involves treating the corresponding Organotin chemistry#Organotin oxides and hydroxides, hydroxide or oxide with a halogenating agent such as hydrogen chloride or thionyl chloride (): : Uses Trimethyltin chloride is used as a source of the trimethylstannyl group. For example, it is a precursor to vinyltrimethylstannane and indenyltrimethylstanane: :CH2=CHMgBr + Me3SnCl → Me3SnCH=CH2 + MgBrCl :LiC9H7 + Me3SnCl → Me3SnC9H7 + LiCl An example of an Organolithium reagent, organolithium reagent reacting with Me3SnCl to form a tin- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tributyltin Chloride
Tributyltin chloride is an organotin compound with the formula ( C4H9)3SnCl. It is a colorless liquid that is soluble in organic solvents. Preparation and reactions The compound is prepared by a redistribution reaction by combining stannic chloride and tetrabutyltin: :3 (C4H9)4Sn + SnCl4 → 4 (C4H9)3SnCl Tributyltin chloride hydrolyzes to the oxide C4H9)3Snsub>2O Tributyltin chloride is used as a precursor to other organotin compounds{{cite journal, title=Palladium-catalyzed Coupling Of Acid Chlorides With Organotin Reagents: Ethyl (E)-4-(4-nitrophenyl)-4-oxo-2-butenoate, authors=A. F. Renaldo, J. W. Labadie, And J. K. Stille, journal=Org. Synth., year=1989, volume=67, page=86, doi=10.15227/orgsyn.067.0086 and reagents, such as tributyltin hydride Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis. Synthesis an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
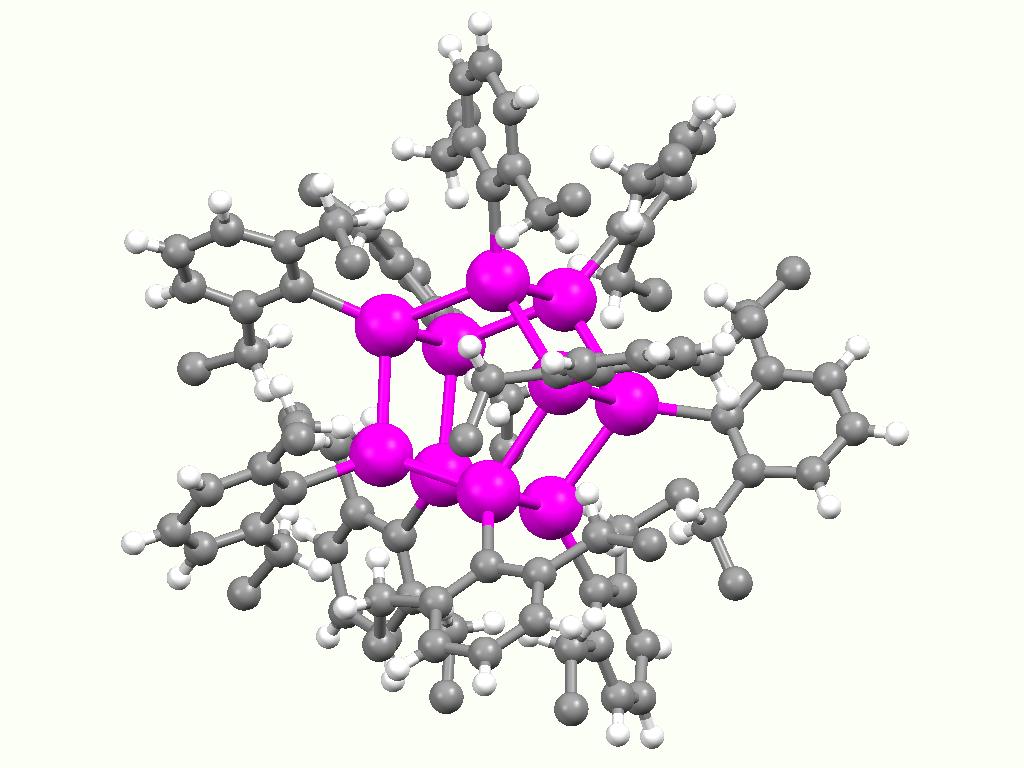
Stannoxane
Stannoxane is a functional group in organotin chemistry with the connectivity SnIV-O-SnIV (IV indicates the oxidation state of tin). Aside from the oxide group, usually 3 or 4 other substituents are attached to tin. In aqueous or aquatic environments, most organotin compounds contain this group.Davies, Alwyn George. (2004) Organotin Chemistry, 2nd Edition Weinheim: Wiley-VCH. : Synthesis and formation Stannoxanes form upon hydrolysis of organotin halides. For example, hydrolysis of dibutyltin dichloride gives the tetratin compound { u2ClSnsub>2O}2. The hydrolysis appears to proceed via organotin hydroxides. For example, the commercially important (C6H11)3SnOH converts at 200 °C into the distannoxane: :2 (C6H11)3SnOH → C6H11)3Snsub>2O + H2O The condensation process is proposed to occur via an associative mechanism Associative substitution describes a pathway by which compounds interchange ligands. The terminology is typically applied to organometallic and coordina ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tributyltin Hydride
Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis. Synthesis and characterization The compound is produced by reduction of tributyltin oxide with polymethylhydrosiloxane: : 2 " eSi(H)Osub>n" + (Bu3Sn)2O → " eSi(OH)Osub>n" + 2 Bu3SnH The hydride is a distillable liquid that is mildly sensitive to air, decomposing to (Bu3Sn)2O. Its IR spectrum exhibits a strong band at 1814 cm−1 for ''ν''Sn−H. Applications It is a specialized reagent in organic synthesis. Combined with azobisisobutyronitrile (AIBN) or by irradiation with light, tributyltin hydride converts organic halides (and related groups) to the corresponding hydrocarbon. This process occurs via a radical chain mechanism involving the radical Bu3Sn•. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tributyltin Hydride
Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis. Synthesis and characterization The compound is produced by reduction of tributyltin oxide with polymethylhydrosiloxane: : 2 " eSi(H)Osub>n" + (Bu3Sn)2O → " eSi(OH)Osub>n" + 2 Bu3SnH The hydride is a distillable liquid that is mildly sensitive to air, decomposing to (Bu3Sn)2O. Its IR spectrum exhibits a strong band at 1814 cm−1 for ''ν''Sn−H. Applications It is a specialized reagent in organic synthesis. Combined with azobisisobutyronitrile (AIBN) or by irradiation with light, tributyltin hydride converts organic halides (and related groups) to the corresponding hydrocarbon. This process occurs via a radical chain mechanism involving the radical Bu3Sn•. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stannane
Stannane or tin hydride is an inorganic compound with the chemical formula . It is a colourless gas and the tin analogue of methane. Stannane can be prepared by the reaction of and . : Stannane decomposes slowly at room temperature to give metallic tin and hydrogen and ignites on contact with air. Variants of stannane can be found as a highly toxic, gaseous, inorganic metal hydride and group 14 hydride. See also * Organotin Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide (), discovered by ... References {{Hydrides by group Tin(IV) compounds Metal hydrides Reducing agents ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polystannane
{{Short description, Family of inorganic polymers containing a tin-tin backbone Polystannanes are organotin compounds with the formula (R2Sn)n. These polymers have been of intermittent academic interest; they are unusual because heavy elements comprise the backbone. Structurally related but better characterized (and more useful) are the polysilanes (R2Si)n. History Oligo- or polystannanes were first described by Löwig in 1852, only 2 years after Edward Frankland's report on the isolation of the first organotin compounds. Löwig' route involved treating an Sn/K and Sn/Na alloys with iodoethane, in the presence of quartz sand which was used to control the reaction rate. Products with elemental compositions close to those of oligo(diethylstannane)s or poly(diethylstannane) were obtained. Cahours obtained similar products and attributed the formation of the so-called "stannic ethyl" to a reaction of the Wurtz type. Already in 1858, "stannic ethyl" was formulated as a polymeric c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Edward Frankland
Sir Edward Frankland, (18 January 18259 August 1899) was an English chemist. He was one of the originators of organometallic chemistry and introduced the concept of combining power or valence. An expert in water quality and analysis, he was a member of the second royal commission on the pollution of rivers, and studied London's water quality for decades. He also studied luminous flames and the effects of atmospheric pressure on dense ignited gas, and was one of the discoverers of helium. Biography Edward Frankland was born in Catterall, Lancashire and baptised at Churchtown, Lancashire on 20 February 1825. As his baptismal record shows, his birth was illegitimate. His mother, Margaret "Peggy" Frankland, later married William Helm, a Lancaster cabinet-maker. "His illegitimacy cast a shadow over all his life since he was pledged to silence as to the identity of his natural father, though a handsome annuity was paid to his mother". From age 3 to 8 Edward lived and was educ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term " metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are repres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes foun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist between two different elements: for example, in a carbonyl group between a carbon atom and an oxygen atom. Other common double bonds are found in azo compounds (N=N), imines (C=N), and sulfoxides (S=O). In a skeletal formula, a double bond is drawn as two parallel lines (=) between the two connected atoms; typographically, the equals sign is used for this. Double bonds were first introduced in chemical notation by Russian chemist Alexander Butlerov. Double bonds involving carbon are stronger and shorter than single bonds. The bond order is two. Double bonds are also electron-rich, which makes them potentially more reactive in the presence of a strong electron acceptor (as in addition reactions of the halogens). File:Ethene structural.svg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |