Germylene Hydride Synthesis From Adduct on:
[Wikipedia]
[Google]
[Amazon]
 Germylenes are a class of
Germylenes are a class of

 Dimerization of free germylenes does not have a noticeable energy barrier, which means that the dimerization reaction is almost
Dimerization of free germylenes does not have a noticeable energy barrier, which means that the dimerization reaction is almost

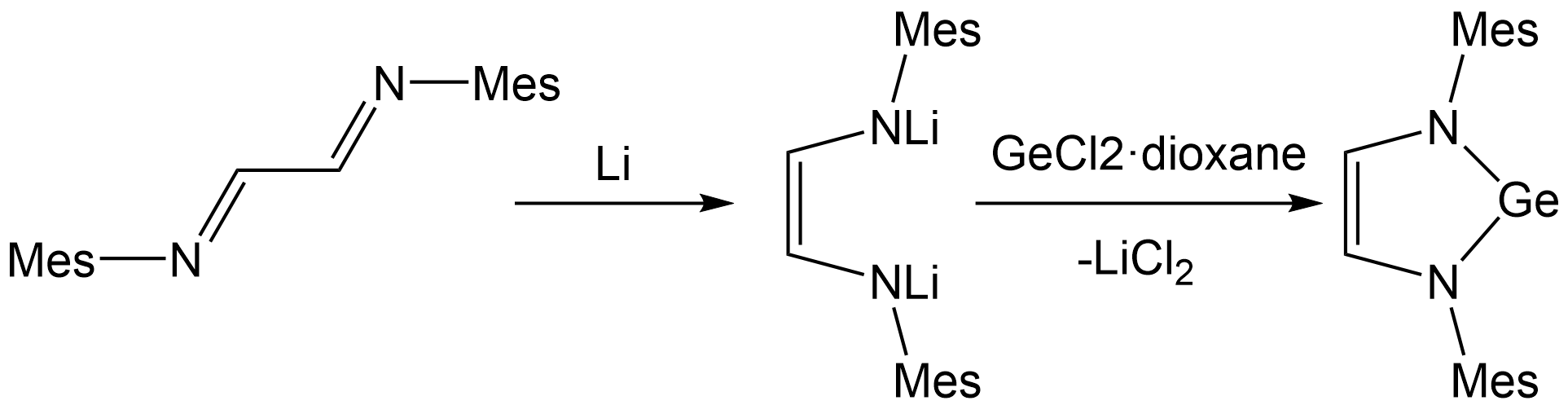 The cyclic(alkyl)(amino)carbenes (CAACs) has already been known as both a better
The cyclic(alkyl)(amino)carbenes (CAACs) has already been known as both a better 


 C-H bonds are generally unreactive toward germylene insertion. However, strain release may help to overcome the activation energy barrier.
C-H bonds are generally unreactive toward germylene insertion. However, strain release may help to overcome the activation energy barrier.
 Insertion to carbon-halide bonds is common for germylene. The mechanism for insertion of free Me2Ge into the C-Br bond of benzyl bromide was reported to be a two-step,
Insertion to carbon-halide bonds is common for germylene. The mechanism for insertion of free Me2Ge into the C-Br bond of benzyl bromide was reported to be a two-step,  The insertion into the C-Hal bond in
The insertion into the C-Hal bond in  For C-O, the R2Ge insertion product could only remain stable at a very low temperature.
For C-O, the R2Ge insertion product could only remain stable at a very low temperature.

 A variety of 1,2-substituted-vinylgermyl compounds can be synthesized in both high yield and high regioselectivity by addition of germylene to alkynes.
A variety of 1,2-substituted-vinylgermyl compounds can be synthesized in both high yield and high regioselectivity by addition of germylene to alkynes.
 1,4-Cycloaddition of conjugated (hetero-) dienes by free germylenes gives the corresponding 5-membered ring.
1,4-Cycloaddition of conjugated (hetero-) dienes by free germylenes gives the corresponding 5-membered ring.
 Germylenes reacts only with one of the strained double bonds in cumulated systems like
Germylenes reacts only with one of the strained double bonds in cumulated systems like

 Germylenes are a class of
Germylenes are a class of germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors s ...
(II) compounds with the general formula :GeR2. They are heavier carbene analogs Carbene analogs in chemistry are carbenes with the carbon atom replaced by another chemical element. Just as regular carbenes they appear in chemical reactions as reactive intermediates and with special precautions they can be stabilized and isolate ...
. However, unlike carbenes, whose ground state can be either singlet or triplet
A triplet is a set of three items, which may be in a specific order, or unordered. It may refer to:
Science
* A series of three nucleotide bases forming an element of the Genetic code
* J-coupling as part of Nuclear magnetic resonance spectrosc ...
depending on the substituents, germylenes have exclusively a singlet ground state. Unprotected carbene analogs, including germylenes, has a dimerization
A dimer () (''wikt:di-, di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, Covalent bond, covalent or Intermolecular force, intermolecular. Dimers also have significant im ...
nature. Free germylenes can be isolated under the stabilization of steric hindrance or electron donation. The synthesis of first stable free dialkyl germylene was reported by Jutzi, et al in 1991.Structures and bonding
Bonding situation for germylene is distinctively different from that for its light analog carbene. The carbon atom from carbene is sp2 hybridized. Although germylenes still have some sp2hybridization
Hybridization (or hybridisation) may refer to:
*Hybridization (biology), the process of combining different varieties of organisms to create a hybrid
*Orbital hybridization, in chemistry, the mixing of atomic orbitals into new hybrid orbitals
*Nu ...
character, the larger energy gap between s and p-orbitals for germanium permits the retainment of 4s24p2 electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
to some degree. The bond angle for H2Ge and Me2Ge was found to be: H-Ge-H 93° and C-Ge-C: 98°, which is smaller than 120°, the ideal bond angle for sp2 hybridized structure and thus proves the 4s24p2 valence electron configuration nature of germylene. The lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
of germylene tends to stay in the high-s-character orbital which is relatively inert
Inert may refer to:
* Chemically inert, not chemically reactive
** Inert gas
** Noble gas, historically called inert gas
* Inert knowledge, information which one can express but not use
* Inert waste, waste which is neither chemically nor biol ...
, making germylene exclusively singlet.
Dimerization of germylenes lead to the formation of germylene dimers (R2Ge=GeR2). It was found that multiple bonds between germanium atoms are not necessarily classical σ and π bonds
In chemistry, pi bonds (π bonds) are covalent bond, covalent chemical chemical bond, bonds, in each of which two lobes of an atomic orbital, orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap oc ...
as found for carbene dimers ( alkenes), but can rather be regarded as donor–acceptor adducts of a trans-bent structure.

Synthesis
Stabilization
 Dimerization of free germylenes does not have a noticeable energy barrier, which means that the dimerization reaction is almost
Dimerization of free germylenes does not have a noticeable energy barrier, which means that the dimerization reaction is almost spontaneous
Spontaneous may refer to:
* Spontaneous abortion
* Spontaneous bacterial peritonitis
* Spontaneous combustion
* Spontaneous declaration
* Spontaneous emission
* Spontaneous fission
* Spontaneous generation
* Spontaneous human combustion
* Spontan ...
and diffusion limited, so the free germylene monomers without stabilization could dimerize or further polymerize once they form. Free germylenes have to be stabilized kinetically or thermodynamically due to their high reactivity originating from the vacant p-orbital. Thermodynamical stabilization of this p-orbital is usually realized by coordination
Coordination may refer to:
* Coordination (linguistics), a compound grammatical construction
* Coordination complex, consisting of a central atom or ion and a surrounding array of bound molecules or ions
* Coordination number or ligancy of a centr ...
of a pentamethylcyclopentadiene (Cp*) ligand or of nitrogen (N), oxygen (O) or phosphorus (P) containing ligands, which are able to donate electrons and thus deactivate the vacant p-orbitals. At the same time, stabilization can be accomplished by steric protection of bulky R groups like mesityl groups (Mes) to prevent nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
s from getting close to the germanium center.Synthesis of carbon substituted germylenes
Carbon substituents is different from other heteroatom N, O, P substituents which have lone pairs in that they provide less electronic perturbations. As a result, a stronger steric and electronic stabilization is required to guarantee a monomeric product. Carbon substituted germylenes can be synthesized using various methods: (1) reduction of dibromogermanes with reducing agents like lithium naphthalene (LiNp) or potassium graphite (KC8), etc., (2)photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
of strained cyclogermanes or Ge(IV) species, (3) substitution of a dihalo Ge(II) precursor species with nucleophiles like organometallic ligands (e.g. RLi/RMgBr).

Synthesis of n-heterocyclic germylene and cyclic(alkyl)(amino)germylene
The introduction of heteroatom in the ligand backbone enhances the stability of reactive Ge(II) center by electron donation from N lone pair to vacant p-orbitals of germanium center. Typically, the strategy for synthesizing five-membered N-heterocyclic tetrylene involves the reaction between N-substituted 1,4-diaza-1,3-butadiene, thealkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
based reducing agents and group 14 halides. In the case of n-heterocyclic germylene (NHGe) synthesis, the method involves an initial reduction of N-substituted 1,4-diaza-1,3-butadiene by lithium. The following cyclization of the dianion with the corresponding Ge(II) halides gives the final product.
 The cyclic(alkyl)(amino)carbenes (CAACs) has already been known as both a better
The cyclic(alkyl)(amino)carbenes (CAACs) has already been known as both a better donor
A donor in general is a person, organization or government which donates something voluntarily. The term is usually used to represent a form of pure altruism, but is sometimes used when the payment for a service is recognized by all parties as rep ...
and better acceptor
Acceptor may refer to:
* Acceptor (accounting), the addressee of a bill of exchange
* In the Indian Contract Act of 1872, the acceptor is the person to whom a proposal is made, and who has communicated his or her acceptance of the said proposal
...
than n-heterocyclic carbenes (NHCs) due to its higher highest occupied molecular orbital (HOMO) and lower lowest unoccupied molecular orbital (LUMO).
The synthetic strategy of CAAGe involves the synthesis of a α-β-unsaturated imine from a ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
and an amine via condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to ...
followed by the treatment with GeCl2· dioxane. The resulting product is then reduced with KC8 to give CAAGe. Analogous to CAAC, the electrophilicity of the germanium center can be obviously enhanced by the substitution of a π-donating and σ-withdrawing amino group along with σ-donating trimethylsilyl groups.

Synthesis of a unique homoconjugation stabilized germylene
In 2016, Muller et al reported the synthesis of a unique homoconjugation stabilized germylene in a relatively high yield by the reaction between hafnocene dichloride and dipotassium germacyclopentadienediide in THF at -80 °C. The product is stabilized by a remote interaction between a C=C double bond and vacant p-orbital of Ge center through homoconjugation. This stabilization strategy results in a special structural which possesses unusual reactivity.
Synthesis of PGeP pincer compounds
The pincer based germylene is of great importance not only for their ability to stabilize transition metal species via chelation effects in homogeneous catalysis, but also for its serving as a good luminescence source. A PNHNHP ligand was used to synthesize the PGeP pincer stabilized germylene by treatment with two equivalents of potassium hexamethyldisilazide (KHMDS) and GeCl2·dioxane, which finally leads to the formation of the PGeP pincer compound.
Reactivity
Oligomerization and polymerization
Dimerization of carbon substituted germylenes gives R2Ge=GeR2 dimers which could further polymerize to form polygermanes (R2Ge)n compounds. The dimer could show a certain stability if prepared in an independent way. Bulkier substituents are able to reduce the polymerization rate by steric effect. More steric hindrance could even stop the polymerization or dimerization reactions and renders a germylene thermodynamically stable.Insertion into σ bond
R2Geinsertion
Insertion may refer to:
*Insertion (anatomy), the point of a tendon or ligament onto the skeleton or other part of the body
*Insertion (genetics), the addition of DNA into a genetic sequence
*Insertion, several meanings in medicine, see ICD-10-PCS
...
into C-C bonds has not been reported so far. However, going down the group 14, C-E (E = Si, Ge, Sn, Pb) bonds become more accessible for R2Ge insertion. The strained C-Ge bonds allow insertion of germylene to 7,7-dialkyl-7-germanorbornadienes in the melt, forming digermabicy-clooctadienes.
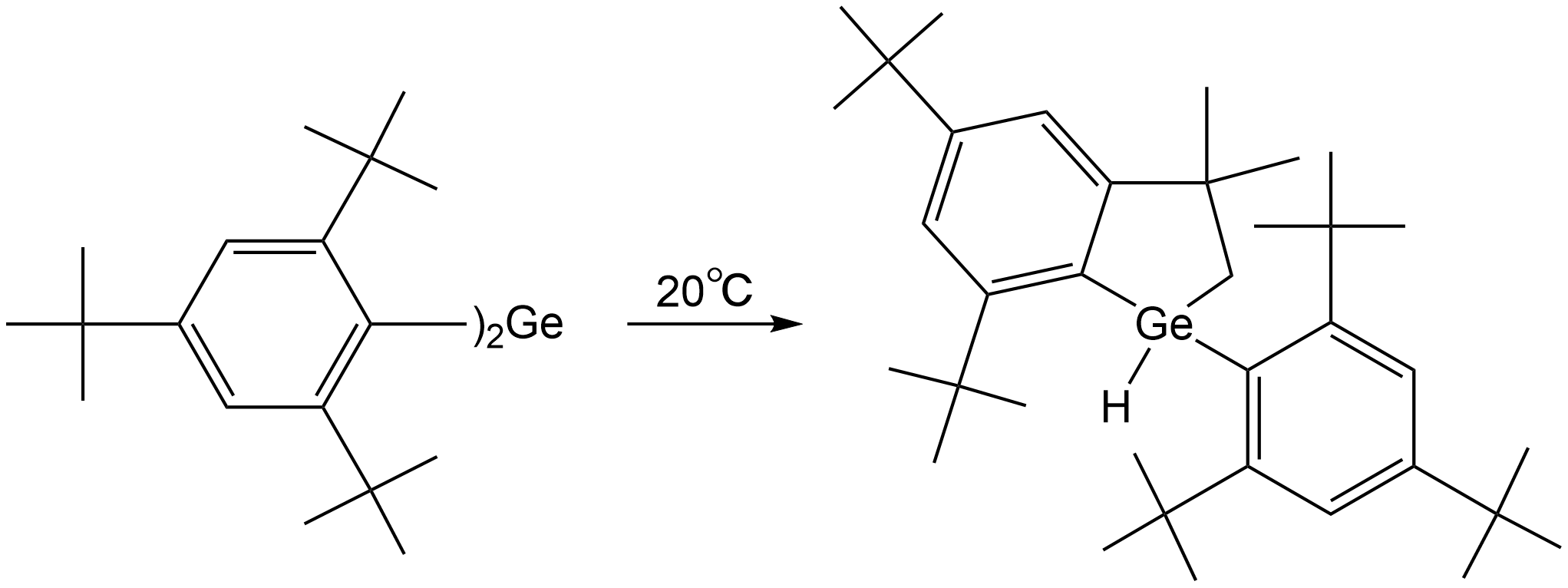
 C-H bonds are generally unreactive toward germylene insertion. However, strain release may help to overcome the activation energy barrier.
C-H bonds are generally unreactive toward germylene insertion. However, strain release may help to overcome the activation energy barrier.
 Insertion to carbon-halide bonds is common for germylene. The mechanism for insertion of free Me2Ge into the C-Br bond of benzyl bromide was reported to be a two-step,
Insertion to carbon-halide bonds is common for germylene. The mechanism for insertion of free Me2Ge into the C-Br bond of benzyl bromide was reported to be a two-step, radical
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
abstraction- recombination process under thermal and photolytical conditions. An identical mechanism through a caged singlet radical pair was proposed for C-Cl bond insertion. However, the interaction between halogen electrons and empty p-orbital of the germylene may result in the formation of a donor-acceptor complex before occurrence of any of the insertion mechanisms.
 The insertion into the C-Hal bond in
The insertion into the C-Hal bond in alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
compounds go by a one-step mechanism under thermal or photolytical conditions.
 For C-O, the R2Ge insertion product could only remain stable at a very low temperature.
For C-O, the R2Ge insertion product could only remain stable at a very low temperature.

Addition to unsaturated systems
Addition reaction of Me2Ge to unsaturated systems is well studied. As mentioned above, dimerization and polymerization of Me2Ge does not have a noticeable activation energy barrier and is only controlled by diffusion. As a result, addition reactions should be rapid enough complete before getting polygermanes as products. There is no reaction between simple alkenes and free germylenes. However,styrenes
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
and α-substituted styrenes are able to react with Me2Ge. The resulting product is a 1:1 mixture of the syn and anti-isomers of 3,4-diphenyl-3,4-R-1,1-dimethyl-1-germacyclopentane. A variety of 1,2-substituted-vinylgermyl compounds can be synthesized in both high yield and high regioselectivity by addition of germylene to alkynes.
A variety of 1,2-substituted-vinylgermyl compounds can be synthesized in both high yield and high regioselectivity by addition of germylene to alkynes.
 1,4-Cycloaddition of conjugated (hetero-) dienes by free germylenes gives the corresponding 5-membered ring.
1,4-Cycloaddition of conjugated (hetero-) dienes by free germylenes gives the corresponding 5-membered ring.
 Germylenes reacts only with one of the strained double bonds in cumulated systems like
Germylenes reacts only with one of the strained double bonds in cumulated systems like allenes
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres (). Allenes are classified as diene#Classes, cumulated dienes. The parent compound of this class is propa ...
(C=C=C). Germylenes prefer to react with more electron-deficient Electron deficiency (and electron-deficient) is jargon that is used in two contexts: species that violate the octet rule because they have too few valence electrons and species that happen to follow the octet rule but have electron-acceptor properti ...
allenes.
Complexation by donors
During complexation with donors, the germylenes stay in the singlet ground state, where the lone pair is placed in the high-s-character orbital, while the heteroatom-containing donors like R2O, ROH, R2S, R3P, R3N and RCl interact with the vacant p-orbital at germanium center, which could stabilize the singlet germylene and prevent further polymerization. Most of the complexes are stable in room temperature. The absorption bands of adducts commonly exhibits shorter wavelengths in comparison to those of the free germylenes due to substituent-influenced n-p transitions at the Ge center.Germylene catalyzed reaction
Germylenes could also act as catalysts as transition metals do. Oxidative addition and reductive elimination, along with the related Mn+/M(n+2)+ redox couples are of great significance to the transition metal catalysis. Due to the electronic structure and chemical properties of germylenes, including the empty p-orbital, germylenes are able to activated small molecules and give the corresponding Ge(IV) complexes, which raised researchers' interests in germylenes' acting asspectator ligands In coordination chemistry, a spectator ligand is a ligand that does not participate in chemical reactions of the complex. Instead, spectator ligands (vs "actor ligands") occupy coordination sites. Spectator ligands tend to be of polydentate, such t ...
in certain catalytic cycles
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials ...
. However, subsequent regeneration of Ge(II) compound through reductive elimination is not thermodynamically favored for germylenes. 1/sup> The key of germylene catalysis chemistry is to maintain a balance between oxidative addition and reductive elimination. One example of germylene catalyzed reaction is hydroboration of CO2, where a preliminary hydrogermylation of CO2 step is followed by the formation of methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
derivatives with 3 equivalent of catecholborane
Catecholborane (abbreviated HBcat) is an organoboron compound that is useful in organic synthesis. This colourless liquid is a derivative of catechol and a borane, having the formula C6H4O2BH.
Synthesis and structure
Traditionally catecholborane ...
to regenerate the germylene compound.{{Cite journal , last1=Hadlington , first1=Terrance J. , last2=Kefalidis , first2=Christos E. , last3=Maron , first3=Laurent , last4=Jones , first4=Cameron , date=2017 , title=Efficient Reduction of Carbon Dioxide to Methanol Equivalents Catalyzed by Two-Coordinate Amido–Germanium(II) and −Tin(II) Hydride Complexes , url=https://pubs.acs.org/doi/10.1021/acscatal.6b03306 , journal=ACS Catalysis , language=en , volume=7 , issue=3 , pages=1853–1859 , doi=10.1021/acscatal.6b03306 , issn=2155-5435

See also
* Carbene analogs *Silylene
Silylene is a chemical compound with the formula SiH2. It is the silicon analog of methylene, the simplest carbene. Silylene is a stable molecule as a gas but rapidly reacts in a bimolecular manner when condensed. Unlike carbenes, which can exis ...
* Stannylene
Stannylenes (R2Sn:) are a class of organotin chemistry, organotin(II) compounds that are analogues of carbene. Unlike carbene, which usually has a triplet ground state, stannylenes have a singlet ground state since valence orbitals of tin (Sn) hav ...
References