|
Stannylene
Stannylenes (R2Sn:) are a class of organotin chemistry, organotin(II) compounds that are analogues of carbene. Unlike carbene, which usually has a triplet ground state, stannylenes have a singlet ground state since valence orbitals of tin (Sn) have less tendency to form hybrid orbitals and thus the electrons in 5s orbital are still paired up. Free stannylenes are stabilized by steric protection. Adducts with Lewis bases are also known. History The first persistent stannylene, [(Me3Si)2CH]2Sn, was reported by Michael F. Lappert in 1973. Lappert used the same synthetic approach to synthesize the first diamidostannylene [(Me3Si)2N]2Sn in 1974. The short-lived, transient stannylene Me2Sn has been generated by thermal decomposition, thermolysis of ''cyclo''-(Me2Sn)6. Synthesis and characterization Persistent stannylene Most alkyl stannylenes have been synthesized by alkylation of tin(II) dihalides with organolithium reagents. For example, the first stannylene, [(Me3Si)2CH]2Sn, was sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stannylene
Stannylenes (R2Sn:) are a class of organotin chemistry, organotin(II) compounds that are analogues of carbene. Unlike carbene, which usually has a triplet ground state, stannylenes have a singlet ground state since valence orbitals of tin (Sn) have less tendency to form hybrid orbitals and thus the electrons in 5s orbital are still paired up. Free stannylenes are stabilized by steric protection. Adducts with Lewis bases are also known. History The first persistent stannylene, [(Me3Si)2CH]2Sn, was reported by Michael F. Lappert in 1973. Lappert used the same synthetic approach to synthesize the first diamidostannylene [(Me3Si)2N]2Sn in 1974. The short-lived, transient stannylene Me2Sn has been generated by thermal decomposition, thermolysis of ''cyclo''-(Me2Sn)6. Synthesis and characterization Persistent stannylene Most alkyl stannylenes have been synthesized by alkylation of tin(II) dihalides with organolithium reagents. For example, the first stannylene, [(Me3Si)2CH]2Sn, was sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
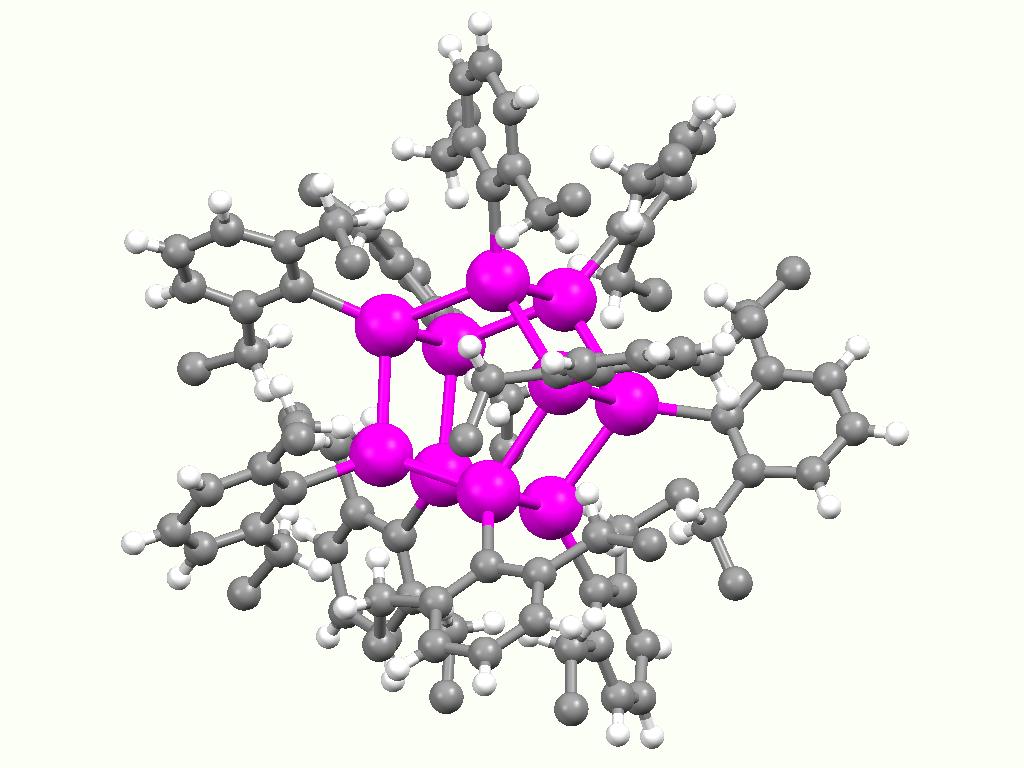
Stannylene Synthesis
Stannylenes (R2Sn:) are a class of organotin(II) compounds that are analogues of carbene. Unlike carbene, which usually has a triplet ground state, stannylenes have a singlet ground state since valence orbitals of tin (Sn) have less tendency to form hybrid orbitals and thus the electrons in 5s orbital are still paired up. Free stannylenes are stabilized by steric protection. Adducts with Lewis bases are also known. History The first persistent stannylene, Me3Si)2CHsub>2Sn, was reported by Michael F. Lappert in 1973. Lappert used the same synthetic approach to synthesize the first diamidostannylene Me3Si)2Nsub>2Sn in 1974. The short-lived, transient stannylene Me2Sn has been generated by thermolysis of ''cyclo''-(Me2Sn)6. Synthesis and characterization Persistent stannylene Most alkyl stannylenes have been synthesized by alkylation of tin(II) dihalides with organolithium reagents. For example, the first stannylene, Me3Si)2CHsub>2Sn, was synthesized using (Me3Si)2CHLi and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stannylene Synthesis Using Organolithium And KC8
Stannylenes (R2Sn:) are a class of organotin(II) compounds that are analogues of carbene. Unlike carbene, which usually has a triplet ground state, stannylenes have a singlet ground state since valence orbitals of tin (Sn) have less tendency to form hybrid orbitals and thus the electrons in 5s orbital are still paired up. Free stannylenes are stabilized by steric protection. Adducts with Lewis bases are also known. History The first persistent stannylene, Me3Si)2CHsub>2Sn, was reported by Michael F. Lappert in 1973. Lappert used the same synthetic approach to synthesize the first diamidostannylene Me3Si)2Nsub>2Sn in 1974. The short-lived, transient stannylene Me2Sn has been generated by thermolysis of ''cyclo''-(Me2Sn)6. Synthesis and characterization Persistent stannylene Most alkyl stannylenes have been synthesized by alkylation of tin(II) dihalides with organolithium reagents. For example, the first stannylene, Me3Si)2CHsub>2Sn, was synthesized using (Me3Si)2CHLi and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Donor-acceptor Interaction
A double is a look-alike or doppelgänger; one person or being that resembles another. Double, The Double or Dubble may also refer to: Film and television * Double (filmmaking), someone who substitutes for the credited actor of a character * ''The Double'' (1934 film), a German crime comedy film * ''The Double'' (1971 film), an Italian film * ''The Double'' (2011 film), a spy thriller film * ''The Double'' (2013 film), a film based on the Dostoevsky novella * ''Kamen Rider Double'', a 2009–10 Japanese television series ** Kamen Rider Double (character), the protagonist in a Japanese television series of the same name Food and drink * Doppio, a double shot of espresso * Dubbel, a strong Belgian Trappist beer or, more generally, a strong brown ale * A drink order of two shots of hard liquor in one glass * A "double decker", a hamburger with two patties in a single bun Games * Double, action in games whereby a competitor raises the stakes ** , in contract bridge ** D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organotin Compounds
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide (), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory. Structure Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful. Organic derivatives of tin(IV) The tetraorgano derivatives are invariably tetrahedral. Compounds of the type SnRR'R''R have been resolved into individual enantiomers. Organotin halides Organotin chlorides have the formula for values of ''n'' up to 3. Bromides, iodides, and fluorides are also known but less important. These ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silylene
Silylene is a chemical compound with the formula SiH2. It is the silicon analog of methylene, the simplest carbene. Silylene is a stable molecule as a gas but rapidly reacts in a bimolecular manner when condensed. Unlike carbenes, which can exist in the singlet or triplet state, silylene (and all of its derivatives) are singlets. Silylenes are formal derivatives of silylene with its hydrogens replaced by other substituents. Most examples feature amido (NR2) or alkyl/aryl groups. Silylenes have been proposed as reactive intermediates. They are carbene analogs. Synthesis and properties Silylenes are generally synthesized by thermolysis or photolysis of polysilanes, by silicon atom reactions ( insertion, addition or abstraction), by pyrolysis of silanes, or by reduction of 1,1-dihalosilane. It has long been assumed that the conversion of metallic Si to tetravalent silicon compounds proceeds via silylene intermediates: :Si + Cl2 → SiCl2 :SiCl2 + Cl2 → SiCl4 Similar considera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbene Analogs
Carbene analogs in chemistry are carbenes with the carbon atom replaced by another chemical element. Just as regular carbenes they appear in chemical reactions as reactive intermediates and with special precautions they can be stabilized and isolated as chemical compounds. Carbenes have some practical utility in organic synthesis but carbene analogs are mostly laboratory curiosities only investigated in academia. Carbene analogs are known for elements of group 13, group 14, group 15 and group 16. Group 13 carbene analogs In group 13 elements the boron carbene analog is called a borylene or boranylidene. Group 14 carbene analogs The heavier group 14 carbenes are silylenes, R2Si:, germylenes R2Ge: (example diphosphagermylene), stannylenes R2Sn: and plumbylenes R2Pb:, collectively known as metallylenes and regarded as monomers for polymetallanes. The oxidation state for these compounds is +2 and stability increases with principal quantum number (moving down a row in the periodic tab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidative addition is often a step in catalytic cycles, in conjunction with its reverse reaction, reductive elimination. Role in transition metal chemistry For transition metals, oxidative reaction results in the decrease in the d''n'' to a configuration with fewer electrons, often 2e fewer. Oxidative addition is favored for metals that are (i) basic and/or (ii) easily oxidized. Metals with a relatively low oxidation state often satisfy one of these requirements, but even high oxidation state metals undergo oxidative addition, as illustrated by the oxidation of Pt(II) with chlorine: : tCl4sup>2− + Cl2 → tCl6sup>2− In classical organometallic chemistry, the formal oxidation state of the metal and the electron count of the complex both in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |