


Nuclear fuel is material used in nuclear power stations to produce heat to power
turbine
A turbine ( or ) (from the Greek , ''tyrbē'', or Latin ''turbo'', meaning vortex) is a rotary mechanical device that extracts energy from a fluid flow and converts it into useful work. The work produced by a turbine can be used for generating ...
s. Heat is created when nuclear fuel undergoes
nuclear fission
Nuclear fission is a nuclear reaction, reaction in which the atomic nucleus, nucleus of an atom splits into two or more smaller atomic nucleus, nuclei. The fission process often produces gamma ray, gamma photons, and releases a very large ...
.
Most nuclear fuels contain heavy
fissile
In nuclear engineering, fissile material is material capable of sustaining a nuclear fission chain reaction. By definition, fissile material can sustain a chain reaction with neutrons of thermal energy. The predominant neutron energy may be typ ...
actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The inf ...
elements that are capable of
undergoing and sustaining nuclear fission. The three most relevant fissile isotopes are
uranium-233
Uranium-233 (233U or U-233) is a fissile isotope of uranium that is bred from thorium-232 as part of the thorium fuel cycle. Uranium-233 was investigated for use in nuclear weapons and as a reactor fuel. It has been used successfully in ex ...
,
uranium-235
Uranium-235 (235U or U-235) is an Isotopes of uranium, isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile ...
and
plutonium-239. When the unstable nuclei of these atoms are hit by a slow-moving neutron, they frequently split, creating two daughter nuclei and two or three more
neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behav ...
s. In that case, the neutrons released go on to split more nuclei. This creates a self-sustaining
chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that sy ...
that is controlled in a
nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nu ...
, or uncontrolled in a
nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion reactions ( thermonuclear bomb), producing a nuclear explosion. Both bom ...
. Alternatively, if the nucleus absorbs the neutron without splitting, it creates a heavier nucleus with one additional neutron.
The processes involved in mining, refining, purifying, using, and disposing of nuclear fuel are collectively known as the
nuclear fuel cycle
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the ''front end'', which are the preparation of the fuel, steps in the ''service period'' in w ...
.
Not all types of nuclear fuels create power from nuclear fission;
plutonium-238
Plutonium-238 (238Pu or Pu-238) is a fissile, radionuclide, radioactive isotope of plutonium that has a half-life of 87.7 years.
Plutonium-238 is a very powerful alpha emitter; as alpha particles are easily blocked, this makes the plutonium-238 ...
and some other elements are used to produce small amounts of nuclear power by
radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
in
radioisotope thermoelectric generator
A radioisotope thermoelectric generator (RTG, RITEG), sometimes referred to as a radioisotope power system (RPS), is a type of nuclear battery that uses an array of thermocouples to convert the heat released by the decay of a suitable radioacti ...
s and other types of
atomic batteries.
Nuclear fuel has the highest
energy density
In physics, energy density is the amount of energy stored in a given system or region of space per unit volume. It is sometimes confused with energy per unit mass which is properly called specific energy or .
Often only the ''useful'' or ex ...
of all practical fuel sources.
Oxide fuel
For fission reactors, the fuel (typically based on uranium) is usually based on the metal oxide; the oxides are used rather than the metals themselves because the oxide melting point is much higher than that of the metal and because it cannot burn, being already in the oxidized state.

Uranium dioxide
Uranium dioxide
Uranium dioxide or uranium(IV) oxide (), also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reac ...
is a black
semiconducting solid. It can be made by heating
uranyl nitrate to form
:
UO2(NO3)2.6H2O-> UO3 + 2NO2 + 1/2O2 + 6H2O(g)
This is then converted by heating with
hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
to form UO
2. It can be made from enriched uranium hexafluoride by reacting with
ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogeno ...
to form a solid called
ammonium diuranate,
(NH4)2U2O7. This is then heated (calcined) to form and U
3O
8 which is then converted by heating with
hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
or ammonia to form UO
2.
The UO
2 is mixed with an organic binder and pressed into pellets, these pellets are then fired at a much higher temperature (in H
2/Ar) to
sinter
Sinter may refer to:
* Sinter plant, in which iron-ore dust gets mixed with other fine materials at high temperature, to create a product – sinter – for use in a blast furnace
* Sintering, a high temperature process for fusing powder together ...
the solid. The aim is to form a dense solid which has few pores.
The thermal conductivity of uranium dioxide is very low compared with that of zirconium metal, and it goes down as the temperature goes up.
Corrosion of uranium dioxide in water is controlled by similar
electrochemical
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
processes to the
galvanic corrosion
Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. A sim ...
of a metal surface.
While exposed to the neutron flux during normal operation in the core environment a small percentage of the Uranium-238 in the fuel absorbs excess neutrons and is transmuted into U-239. U-239 rapidly decays into Neptunium-239 which in turn rapidly decays into Plutonium-239. The small percentage of Plutonium-239 has a higher neutron cross section than Uranium-235. As the Plutonium-239 accumulates the chain reaction shifts from pure Uranium-235 at initiation of the fuel use to a ratio of about 70% Uranium-235 and 30% Plutonium-239 at the end of the 18 to 24 month fuel exposure period.
MOX
Mixed oxide, or MOX fuel, is a blend of
plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhib ...
and natural or
depleted uranium
Depleted uranium (DU; also referred to in the past as Q-metal, depletalloy or D-38) is uranium with a lower content of the fissile isotope than natural uranium.: "Depleted uranium possesses only 60% of the radioactivity of natural uranium, ...
which behaves similarly (though not identically) to the enriched uranium feed for which most
nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nu ...
s were designed. MOX fuel is an alternative to low enriched uranium (LEU) fuel used in the
light water reactor
The light-water reactor (LWR) is a type of thermal-neutron reactor that uses normal water, as opposed to heavy water, as both its coolant and neutron moderator; furthermore a solid form of fissile elements is used as fuel. Thermal-neutron reac ...
s which predominate
nuclear power
Nuclear power is the use of nuclear reactions to produce electricity. Nuclear power can be obtained from nuclear fission, nuclear decay and nuclear fusion reactions. Presently, the vast majority of electricity from nuclear power is produced ...
generation.
Some concern has been expressed that used MOX cores will introduce new disposal challenges, though MOX is itself a means to dispose of surplus plutonium by
transmutation
Transmutation may refer to:
Pseudoscience and science Alchemy
*Chrysopoeia and argyropoeia, the turning of inexpensive metals, such as lead or copper, into gold and silver
* Magnum opus (alchemy), the creation of the philosopher's stone
* Menta ...
.
Reprocessing of commercial nuclear fuel to make MOX was done in the
Sellafield MOX Plant (England). As of 2015, MOX fuel is made in France (see
Marcoule Nuclear Site), and to a lesser extent in Russia (see
Mining and Chemical Combine), India and Japan. China plans to develop
fast breeder reactors (see
CEFR) and reprocessing.
The
Global Nuclear Energy Partnership, was a U.S. proposal in the
George W. Bush Administration to form an international partnership to see spent nuclear fuel reprocessed in a way that renders the plutonium in it usable for nuclear fuel but not for
nuclear weapons
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion reactions ( thermonuclear bomb), producing a nuclear explosion. Both bom ...
. Reprocessing of spent commercial-reactor nuclear fuel has not been permitted in the United States due to nonproliferation considerations. All of the other reprocessing nations have long had nuclear weapons from military-focused "research"-reactor fuels except for Japan. Normally, with the fuel being changed every three years or so, about half of the Pu-239 is 'burned' in the reactor, providing about one third of the total energy. It behaves like U-235 and its fission releases a similar amount of energy. The higher the burn-up, the more plutonium in the spent fuel, but the lower the fraction of fissile plutonium. Typically about one percent of the used fuel discharged from a reactor is plutonium, and some two thirds of this is fissile (c. 50% Pu-239, 15% Pu-241). Worldwide, some 70 tonnes of plutonium contained in used fuel is removed when refueling reactors each year.
Metal fuel
Metal fuels have the advantage of a much higher heat conductivity than oxide fuels but cannot survive equally high temperatures. Metal fuels have a long history of use, stretching from the
Clementine reactor in 1946 to many test and research reactors. Metal fuels have the potential for the highest fissile atom density. Metal fuels are normally alloyed, but some metal fuels have been made with pure uranium metal. Uranium alloys that have been used include uranium aluminum, uranium zirconium, uranium silicon, uranium molybdenum, and uranium zirconium hydride (UZrH). Any of the aforementioned fuels can be made with plutonium and other actinides as part of a closed nuclear fuel cycle. Metal fuels have been used in water reactors and liquid metal fast breeder reactors, such as
EBR-II.
TRIGA fuel
TRIGA fuel is used in TRIGA (Training, Research, Isotopes,
General Atomics
General Atomics is an American energy and defense corporation headquartered in San Diego, California, specializing in research and technology development. This includes physics research in support of nuclear fission and nuclear fusion energy. Th ...
) reactors.
The TRIGA reactor uses UZrH fuel, which has a prompt negative
fuel temperature coefficient of reactivity, meaning that as the temperature of the core increases, the reactivity decreases—so it is highly unlikely for a meltdown to occur. Most cores that use this fuel are "high leakage" cores where the excess leaked neutrons can be utilized for research. That is, they can be used as a
neutron source
A neutron source is any device that emits neutrons, irrespective of the mechanism used to produce the neutrons. Neutron sources are used in physics, engineering, medicine, nuclear weapons, petroleum exploration, biology, chemistry, and nuclear p ...
. TRIGA fuel was originally designed to use
highly enriched uranium
Enriched uranium is a type of uranium in which the percent composition of uranium-235 (written 235U) has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238 (238 ...
, however in 1978 the U.S. Department of Energy launched its Reduced Enrichment for Research Test Reactors program, which promoted reactor conversion to low-enriched uranium fuel. A total of 35 TRIGA reactors have been installed at locations across the US. A further 35 reactors have been installed in other countries.
Actinide fuel
In a
fast neutron reactor, the minor actinides produced by neutron capture of uranium and plutonium can be used as fuel. Metal actinide fuel is typically an alloy of zirconium, uranium, plutonium, and
minor actinides. It can be made inherently safe as thermal expansion of the metal alloy will increase neutron leakage.
Molten plutonium
Molten plutonium, alloyed with other metals to lower its melting point and encapsulated in tantalum, was tested in two experimental reactors, LAMPRE I and LAMPRE II, at
Los Alamos National Laboratory
Los Alamos National Laboratory (often shortened as Los Alamos and LANL) is one of the sixteen research and development laboratories of the United States Department of Energy (DOE), located a short distance northwest of Santa Fe, New Mexico, i ...
in the 1960s. "LAMPRE experienced three separate fuel failures during operation."
Non-oxide ceramic fuels
Ceramic
A ceramic is any of the various hard, brittle, heat-resistant and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelai ...
fuels other than oxides have the advantage of high heat conductivities and melting points, but they are more prone to
swelling than oxide fuels and are not understood as well.
Uranium nitride
This is often the fuel of choice for reactor designs that
NASA
The National Aeronautics and Space Administration (NASA ) is an independent agency of the US federal government responsible for the civil space program, aeronautics research, and space research.
NASA was established in 1958, succeedi ...
produces, one advantage is that UN has a better
thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
than UO
2. Uranium nitride has a very high melting point. This fuel has the disadvantage that unless
15N was used (in place of the more common
14N) that a large amount of
14C would be generated from the nitrogen by the (n,p)
reaction
Reaction may refer to a process or to a response to an action, event, or exposure:
Physics and chemistry
*Chemical reaction
*Nuclear reaction
*Reaction (physics), as defined by Newton's third law
* Chain reaction (disambiguation).
Biology and me ...
. As the
nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seve ...
required for such a fuel would be so expensive it is likely that the fuel would have to be reprocessed by
pyroprocessing to enable the
15N to be recovered. It is likely that if the fuel was processed and dissolved in
nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
that the nitrogen
enriched with
15N would be diluted with the common
14N.
Fluoride volatility
Fluoride volatility is the tendency of highly fluorinated molecules to vaporize at comparatively low temperatures. Heptafluorides, hexafluorides and pentafluorides have much lower boiling points than the lower- valence fluorides. Most difluoride ...
is a method of reprocessing that does not rely on nitric acid, but it has only been demonstrated in relatively small scale installations whereas the established
PUREX
PUREX (plutonium uranium reduction extraction) is a chemistry, chemical method used to purify fuel for nuclear reactors or nuclear weapons. PUREX is the ''de facto'' standard aqueous nuclear reprocessing method for the recovery of uranium and p ...
process is used commercially for about a third of all spent nuclear fuel (the rest being largely subject to a "once through fuel cycle"). All nitrogen-fluoride compounds are volatile or gaseous at room temperature and could be
fractionally distilled from the other gaseous products (including recovered
uranium hexafluoride) to recover the initially used nitrogen. If the fuel could be processed in such a way as to ensure low contamination with non-radioactive carbon (not a common fission product and absent in nuclear reactors that don't
use it as a moderator) then Fluoride volatility could be used to separate the produced by producing
carbon tetrafluoride. is proposed for use in particularly long lived low power
nuclear batteries called
diamond battery.
Uranium carbide
Much of what is known about uranium carbide is in the form of pin-type fuel elements for
liquid metal fast reactors during their intense study during the 1960s and 1970s. However, recently there has been a revived interest in uranium carbide in the form of plate fuel and most notably, micro fuel particles (such as
TRISO
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergoing ...
particles).
The high thermal conductivity and high melting point makes uranium carbide an attractive fuel. In addition, because of the absence of oxygen in this fuel (during the course of irradiation, excess gas pressure can build from the formation of O
2 or other gases) as well as the ability to complement a ceramic coating (a ceramic-ceramic interface has structural and chemical advantages), uranium carbide could be the ideal fuel candidate for certain
Generation IV reactors
Generation IV reactors (Gen IV) are six nuclear reactor designs recognized by the Generation IV International Forum. The designs target improved safety, sustainability, efficiency, and cost.
The most developed Gen IV reactor design is the sodiu ...
such as the
gas-cooled fast reactor. While the neutron cross section of carbon is low, during years of
burnup
In nuclear power technology, burnup (also known as fuel utilization) is a measure of how much energy is extracted from a primary nuclear fuel source. It is measured as the fraction of fuel atoms that underwent fission in %FIMA (fissions per init ...
, the predominantly will undergo neutron capture to produce stable as well as radioactive . Unlike the produced by using Uranium nitrate, the will make up only a small isotopic impurity in the overall carbon content and thus make the entirety of the carbon content unsuitable for non-nuclear uses but the concentration will be too low for use in nuclear batteries without enrichment.
Nuclear graphite
Nuclear graphite is any grade of graphite, usually synthetic graphite, manufactured for use as a moderator or reflector within a nuclear reactor. Graphite is an important material for the construction of both historical and modern nuclear reactors ...
discharged from reactors where it was used as a moderator presents the same issue.
Liquid fuels
Liquid fuels are liquids containing dissolved nuclear fuel and have been shown to offer numerous operational advantages compared to traditional solid fuel approaches.
Liquid-fuel reactors offer significant safety advantages due to their inherently stable "self-adjusting" reactor dynamics. This provides two major benefits:
- virtually eliminating the possibility of a run-away reactor meltdown,
- providing an automatic load-following capability which is well suited to electricity generation and high-temperature industrial heat applications.
Another major advantage of some liquid core designs is their ability to be drained rapidly into a passively safe dump-tank. This advantage was conclusively demonstrated repeatedly as part of a weekly shutdown procedure during the highly successful 4 year
Molten Salt Reactor Experiment.
Another huge advantage of the liquid core is its ability to release xenon gas, which normally acts as a neutron absorber ( is the strongest known
neutron poison and is produced both directly and as a decay product of as a
fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
) and causes structural occlusions in solid fuel elements (leading to the early replacement of solid fuel rods with over 98% of the nuclear fuel unburned, including many long-lived actinides). In contrast, Molten Salt Reactors (MSR) are capable of retaining the fuel mixture for significantly extended periods, which not only increases fuel efficiency dramatically but also incinerates the vast majority of its own waste as part of the normal operational characteristics. A downside to letting the escape instead of allowing it to capture neutrons converting it to the basically stable and chemically inert , is that it will quickly decay to the highly chemically reactive long lived radioactive , which behaves similar to other
alkali metals and can be taken up by organisms in their metabolism.
Molten salts
Molten salt fuels are mixtures of actinide salts (e.g. thorium/uranium fluoride/chloride) with other salts, used in liquid form above their typical melting points of several hundred degrees C. In some
molten salt-fueled reactor designs, such as the
liquid fluoride thorium reactor (LFTR), this fuel salt is also the coolant; in other designs, such as the
stable salt reactor
The Stable Salt Reactor (SSR) is a nuclear reactor design under development by Moltex Energy Canada Inc. and its subsidiary Moltex Energy USA LLC, based in Canada, the United States, and the United Kingdom, as well as MoltexFLEX Ltd., based in the ...
, the fuel salt is contained in fuel pins and the coolant is a separate, non-radioactive salt. There is a further category of molten salt-cooled reactors in which the fuel is not in molten salt form, but a molten salt is used for cooling.
Molten salt fuels were used in the LFTR known as the
Molten Salt Reactor Experiment, as well as other liquid core reactor experiments. The liquid fuel for the molten salt reactor was a mixture of lithium, beryllium, thorium and uranium fluorides: LiF-BeF
2-ThF
4-UF
4 (72-16-12-0.4 mol%). It had a peak
operating temperature
An operating temperature is the allowable temperature range of the local ambient environment at which an electrical or mechanical device operates. The device will operate effectively within a specified temperature range which varies based on the de ...
of 705 °C in the experiment, but could have operated at much higher temperatures since the boiling point of the molten salt was in excess of 1400 °C.
Aqueous solutions of uranyl salts
The
aqueous homogeneous reactors (AHRs) use a solution of
uranyl sulfate or other uranium salt in water. Historically, AHRs have all been small
research reactor
Research reactors are nuclear fission-based nuclear reactors that serve primarily as a neutron source. They are also called non-power reactors, in contrast to power reactors that are used for electricity production, heat generation, or marit ...
s, not large power reactors. An AHR known as the Medical Isotope Production System is being considered for production of
medical isotopes
A medical isotope is an isotope used in medicine.
The first uses of isotopes in medicine were in radiopharmaceuticals, and this is still the most common use. However more recently, separated stable isotopes have also come into use.
Examples of ...
.
Liquid metals or alloys
The
Dual fluid reactor has a variant DFR/m which works with
eutectic liquid metal alloys, e.g. U-Cr or U-Fe.
Common physical forms of nuclear fuel
Uranium dioxide (UO
2) powder is compacted to cylindrical pellets and sintered at high temperatures to produce ceramic nuclear fuel pellets with a high density and well defined physical properties and chemical composition. A grinding process is used to achieve a uniform cylindrical geometry with narrow tolerances. Such fuel pellets are then stacked and filled into the metallic tubes. The metal used for the tubes depends on the design of the reactor. Stainless steel was used in the past, but most reactors now use a
zirconium alloy
Zirconium alloys are solid solutions of zirconium or other metals, a common subgroup having the trade mark Zircaloy. Zirconium has very low absorption cross-section of thermal neutrons, high hardness, ductility and corrosion resistance. One o ...
which, in addition to being highly corrosion-resistant, has low neutron absorption. The tubes containing the fuel pellets are sealed: these tubes are called fuel rods. The finished fuel rods are grouped into fuel assemblies that are used to build up the core of a power reactor.
Cladding is the outer layer of the fuel rods, standing between the coolant and the nuclear fuel. It is made of a
corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engi ...
-resistant material with low
absorption cross section for
thermal neutrons, usually
Zircaloy or
steel in modern constructions, or
magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
with small amount of aluminium and other metals for the now-obsolete
Magnox reactors. Cladding prevents radioactive fission fragments from escaping the fuel into the coolant and contaminating it. Besides the prevention of radioactive leaks this also serves to keep the coolant as non-corrosive as feasible and to prevent reactions between chemically aggressive fission products and the coolant. (e.g. The highly reactive
alkali metal Caesium
Caesium ( IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that ...
which reacts strongly with water, producing hydrogen, and which is among the more common fission products)
Image:Fuel Pellet.jpg, Nuclear Regulatory Commission
The Nuclear Regulatory Commission (NRC) is an independent agency of the United States government tasked with protecting public health and safety related to nuclear energy. Established by the Energy Reorganization Act of 1974, the NRC began oper ...
(NRC) photo of unirradiated (fresh) fuel pellets.
Image:Pellet rod.jpg, NRC photo of fresh fuel pellets ready for assembly.
Image:Nuclear-Fuel.jpg, NRC photo of fresh fuel assemblies being inspected.

PWR fuel
Pressurized water reactor
A pressurized water reactor (PWR) is a type of light-water nuclear reactor. PWRs constitute the large majority of the world's nuclear power plants (with notable exceptions being the UK, Japan and Canada). In a PWR, the primary coolant (water) is ...
(PWR) fuel consists of cylindrical rods put into bundles. A uranium oxide ceramic is formed into pellets and inserted into
Zircaloy tubes that are bundled together. The Zircaloy tubes are about in diameter, and the fuel cladding gap is filled with
helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
gas to improve
heat conduction from the fuel to the cladding. There are about 179–264 fuel rods per fuel bundle and about 121 to 193 fuel bundles are loaded into a reactor core. Generally, the fuel bundles consist of fuel rods bundled 14×14 to 17×17. PWR fuel bundles are about long. In PWR fuel bundles,
control rod
Control rods are used in nuclear reactors to control the rate of fission of the nuclear fuel – uranium or plutonium. Their compositions include chemical elements such as boron, cadmium, silver, hafnium, or indium, that are capable of absorbing ...
s are inserted through the top directly into the fuel bundle. The fuel bundles usually are enriched several percent in
235U. The uranium oxide is dried before inserting into the tubes to try to eliminate moisture in the ceramic fuel that can lead to corrosion and
hydrogen embrittlement
Hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC), is a reduction in the ductility of a metal due to absorbed hydrogen. Hydrogen atoms are small and can permeate solid metals. Once absorbe ...
. The Zircaloy tubes are pressurized with helium to try to minimize pellet-cladding interaction which can lead to fuel rod failure over long periods.
BWR fuel
In
boiling water reactors (BWR), the fuel is similar to PWR fuel except that the bundles are "canned". That is, there is a thin tube surrounding each bundle. This is primarily done to prevent local
density variations from affecting neutronics and thermal hydraulics of the reactor core. In modern BWR fuel bundles, there are either 91, 92, or 96 fuel rods per assembly depending on the manufacturer. A range between 368 assemblies for the smallest and 800 assemblies for the largest BWR in the U.S. form the reactor core. Each BWR fuel rod is backfilled with helium to a pressure of about .

CANDU fuel
CANDU fuel bundles are about long and in diameter. They consist of sintered (UO
2) pellets in zirconium alloy tubes, welded to zirconium alloy end plates. Each bundle weighs roughly , and a typical core loading is on the order of 4500–6500 bundles, depending on the design. Modern types typically have 37 identical fuel pins radially arranged about the long axis of the bundle, but in the past several different configurations and numbers of pins have been used. The
CANFLEX bundle has 43 fuel elements, with two element sizes. It is also about 10 cm (4 inches) in diameter, 0.5 m (20 in) long and weighs about 20 kg (44 lb) and replaces the 37-pin standard bundle. It has been designed specifically to increase fuel performance by utilizing two different pin diameters. Current CANDU designs do not need enriched uranium to achieve criticality (due to the lower
neutron absorption
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons ...
in their
heavy water moderator
Moderator may refer to:
Government
*Moderator (town official), elected official who presides over the Town Meeting form of government Internet
*Internet forum#Moderators, Internet forum moderator, a person given special authority to enforce the ...
compared to light water), however, some newer concepts call for low enrichment to help reduce the size of the reactors. The
Atucha nuclear power plant
The Atucha Nuclear Complex, or Atucha Nuclear Power Plant, is the location for two adjacent nuclear power plants in Lima, Buenos Aires, Lima, Zárate Partido, Zárate, Buenos Aires Province, about from Buenos Aires, on the right-hand shore of ...
in Argentina, a similar design to the CANDU but built by German
KWU was originally designed for non-enriched fuel but since switched to slightly enriched fuel with a content about 0.1
percentage point
A percentage point or percent point is the unit for the arithmetic difference between two percentages. For example, moving up from 40 percent to 44 percent is an increase of 4 percentage points, but a 10-percent increase in the quantity being me ...
s higher than in natural uranium.
Less-common fuel forms
Various other nuclear fuel forms find use in specific applications, but lack the widespread use of those found in BWRs, PWRs, and CANDU power plants. Many of these fuel forms are only found in research reactors, or have military applications.

Magnox fuel
Magnox (magnesium non-oxidising) reactors are pressurised,
carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
–cooled,
graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on la ...
-
moderated reactors using
natural uranium
Natural uranium (NU or Unat) refers to uranium with the same isotopic ratio as found in nature. It contains 0.711% uranium-235, 99.284% uranium-238, and a trace of uranium-234 by weight (0.0055%). Approximately 2.2% of its radioactivity comes ...
(i.e. unenriched) as fuel and
Magnox alloy as fuel cladding. Working pressure varies from for the steel pressure vessels, and the two
reinforced concrete designs operated at . Magnox alloy consists mainly of
magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
with small amounts of
aluminium
Aluminium (aluminum in AmE, American and CanE, Canadian English) is a chemical element with the Symbol (chemistry), symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately o ...
and other metals—used in cladding unenriched
uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly ...
metal fuel with a non-oxidising covering to contain fission products. This material has the advantage of a low
neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behav ...
capture cross-section, but has two major disadvantages:
* It limits the maximum temperature, and hence the
thermal efficiency
In thermodynamics, the thermal efficiency (\eta_) is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc.
For a ...
, of the plant.
* It reacts with water, preventing long-term storage of spent fuel under water - such as in a
spent fuel pool
Spent fuel pools (SFP) are storage pools (or "ponds" in the United Kingdom) for spent fuel from nuclear reactors. They are typically 40 or more feet (12 m) deep, with the bottom 14 feet (4.3 m) equipped with storage racks designed to hold ...
.
Magnox fuel incorporated cooling fins to provide maximum heat transfer despite low operating temperatures, making it expensive to produce. While the use of uranium metal rather than oxide made
nuclear reprocessing
Nuclear reprocessing is the chemical separation of fission products and actinides from spent nuclear fuel. Originally, reprocessing was used solely to extract plutonium for producing nuclear weapons. With commercialization of nuclear power, ...
more straightforward and therefore cheaper, the need to reprocess fuel a short time after removal from the reactor meant that the fission product hazard was severe. Expensive
remote handling facilities were required to address this issue.
TRISO fuel
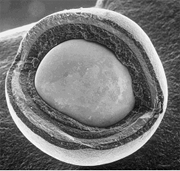
Tristructural-isotropic (TRISO) fuel is a type of micro-particle fuel. A particle consists of a kernel of
UOX fuel (sometimes
UC or UCO), which has been coated with four layers of three
isotropic materials deposited through fluidized
chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (subst ...
(FCVD). The four layers are a porous buffer layer made of carbon that absorbs fission product recoils, followed by a dense inner layer of protective
pyrolytic carbon (PyC), followed by a ceramic layer of
SiC to retain fission products at elevated temperatures and to give the TRISO particle more structural integrity, followed by a dense outer layer of PyC. TRISO particles are then encapsulated into cylindrical or spherical graphite pellets. TRISO fuel particles are designed not to crack due to the stresses from processes (such as differential thermal expansion or fission gas pressure) at temperatures up to 1600 °C, and therefore can contain the fuel in the worst of accident scenarios in a properly designed reactor. Two such reactor designs are the prismatic-block gas-cooled reactor (such as the
GT-MHR) and the
pebble-bed reactor
The pebble-bed reactor (PBR) is a design for a graphite-moderated, gas-cooled nuclear reactor. It is a type of very-high-temperature reactor (VHTR), one of the six classes of nuclear reactors in the Generation IV initiative.
The basic desig ...
(PBR). Both of these reactor designs are
high temperature gas reactor
A high-temperature gas-cooled reactor (HTGR), is a nuclear reactor that uses a graphite-moderated reactor, graphite moderator with a once-through uranium fuel cycle. The HTGR is a type of high-temperature reactor (HTR) that can conceptually have ...
s (HTGRs). These are also the basic reactor designs of
very-high-temperature reactors (VHTRs), one of the six classes of reactor designs in the
Generation IV initiative that is attempting to reach even higher HTGR outlet temperatures.
TRISO fuel particles were originally developed in the United Kingdom as part of the
Dragon reactor project. The inclusion of the SiC as diffusion barrier was first suggested by D. T. Livey. The first nuclear reactor to use TRISO fuels was the Dragon reactor and the first powerplant was the
THTR-300. Currently, TRISO fuel compacts are being used in some experimental reactors, such as the
HTR-10 in China and the
High-temperature engineering test reactor in Japan. Spherical fuel elements utilizing a TRISO particle with a
UO2 and
UC solid solution kernel are being used in the
Xe-100 in the United States.
QUADRISO fuel

In QUADRISO particles a
burnable neutron poison (
europium oxide or
erbium oxide
Erbium(III) oxide is the inorganic compound with the formula . It is a pink paramagnetic solid. It finds uses in various optical materials.
Structure
Erbium(III) oxide has a cubic structure resembling the bixbyite motif. The Er3+ centers are ...
or
carbide
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
Interstitial / Metallic carbides
The carbides of th ...
) layer surrounds the fuel kernel of ordinary TRISO particles to better manage the excess of reactivity. If the core is equipped both with TRISO and QUADRISO fuels, at beginning of life neutrons do not reach the fuel of the QUADRISO particles because they are stopped by the burnable poison. During reactor operation, neutron irradiation of the poison causes it to "burn up" or progressively transmute to non-poison isotopes, depleting this poison effect and leaving progressively more neutrons available for sustaining the chain-reaction. This mechanism compensates for the accumulation of undesirable neutron poisons which are an unavoidable part of the fission products, as well as normal fissile fuel "burn up" or depletion. In the generalized QUADRISO fuel concept the poison can eventually be mixed with the fuel kernel or the outer pyrocarbon. The QUADRISO concept was conceived at
Argonne National Laboratory
Argonne National Laboratory is a science and engineering research national laboratory operated by UChicago Argonne LLC for the United States Department of Energy. The facility is located in Lemont, Illinois, outside of Chicago, and is the lar ...
.

RBMK fuel
RBMK reactor fuel was used in
Soviet
The Soviet Union,. officially the Union of Soviet Socialist Republics. (USSR),. was a transcontinental country that spanned much of Eurasia from 1922 to 1991. A flagship communist state, it was nominally a federal union of fifteen national ...
-designed and built
RBMK-type reactors. This is a low-enriched uranium oxide fuel. The fuel elements in an RBMK are 3 m long each, and two of these sit back-to-back on each fuel channel, pressure tube. Reprocessed uranium from Russian VVER reactor spent fuel is used to fabricate RBMK fuel. Following the Chernobyl accident, the enrichment of fuel was changed from 2.0% to 2.4%, to compensate for control rod modifications and the introduction of additional absorbers.
CerMet fuel
CerMet fuel consists of ceramic fuel particles (usually uranium oxide) embedded in a metal matrix. It is hypothesized that this type of fuel is what is used in United States Navy reactors. This fuel has high heat transport characteristics and can withstand a large amount of expansion.
Plate-type fuel

Plate-type fuel has fallen out of favor over the years. Plate-type fuel is commonly composed of enriched uranium sandwiched between metal cladding. Plate-type fuel is used in several research reactors where a high neutron flux is desired, for uses such as material irradiation studies or isotope production, without the high temperatures seen in ceramic, cylindrical fuel. It is currently used in the
Advanced Test Reactor (ATR) at
Idaho National Laboratory
Idaho National Laboratory (INL) is one of the national laboratories of the United States Department of Energy and is managed by the Battelle Energy Alliance. While the laboratory does other research, historically it has been involved with nu ...
, and the nuclear research reactor at the
University of Massachusetts Lowell Radiation Laboratory.
Sodium-bonded fuel
Sodium-bonded fuel consists of fuel that has liquid sodium in the gap between the fuel slug (or pellet) and the cladding. This fuel type is often used for sodium-cooled liquid metal fast reactors. It has been used in EBR-I, EBR-II, and the FFTF. The fuel slug may be metallic or ceramic. The sodium bonding is used to reduce the temperature of the fuel.
Accident tolerant fuels
Accident tolerant fuels (ATF) are a series of new nuclear fuel concepts, researched in order to improve fuel performance under accident conditions, such as
loss-of-coolant accident (LOCA) or reaction-initiated accidents (RIA). These concerns became more prominent after the
Fukushima Daiichi nuclear disaster in Japan, in particular regarding
light-water reactor
The light-water reactor (LWR) is a type of thermal-neutron reactor that uses normal water, as opposed to heavy water, as both its coolant and neutron moderator; furthermore a solid form of fissile elements is used as fuel. Thermal-neutron reac ...
(LWR) fuels performance under accident conditions.
The aim of the research is to develop nuclear fuels that can tolerate loss of active
cooling for a considerably longer period than the existing fuel designs and prevent or delay the release of
radionuclide
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transfer ...
s during an accident. This research is focused on reconsidering the design of fuel pellets and cladding, as well as the interactions between the two.
Spent nuclear fuel
Used nuclear fuel is a complex mixture of the
fission products
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the relea ...
,
uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly ...
,
plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhib ...
, and the
transplutonium metals. In fuel which has been used at high temperature in power reactors it is common for the fuel to be ''heterogeneous''; often the fuel will contain nanoparticles of
platinum group
The platinum-group metals (abbreviated as the PGMs; alternatively, the platinoids, platinides, platidises, platinum group, platinum metals, platinum family or platinum-group elements (PGEs)) are six noble, precious metallic elements clustered to ...
metals such as
palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself nam ...
. Also the fuel may well have cracked, swollen, and been heated close to its melting point. Despite the fact that the used fuel can be cracked, it is very insoluble in water, and is able to retain the vast majority of the
actinides
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
and
fission products
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the relea ...
within the
uranium dioxide
Uranium dioxide or uranium(IV) oxide (), also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reac ...
crystal lattice
In geometry and crystallography, a Bravais lattice, named after , is an infinite array of discrete points generated by a set of discrete translation operations described in three dimensional space by
: \mathbf = n_1 \mathbf_1 + n_2 \mathbf_2 + n ...
. The radiation hazard from spent nuclear fuel declines as its radioactive components decay, but remains high for many years. For example 10 years after removal from a reactor, the surface dose rate for a typical spent fuel assembly still exceeds 10,000 rem/hour, resulting in a fatal dose in just minutes.
Oxide fuel under accident conditions
Two main modes of release exist, the fission products can be vaporised or small particles of the fuel can be dispersed.
Fuel behavior and post-irradiation examination
Post-Irradiation Examination (PIE) is the study of used nuclear materials such as nuclear fuel. It has several purposes. It is known that by examination of used fuel that the failure modes which occur during normal use (and the manner in which the fuel will behave during an accident) can be studied. In addition information is gained which enables the users of fuel to assure themselves of its quality and it also assists in the development of new fuels. After major accidents the core (or what is left of it) is normally subject to PIE to find out what happened. One site where PIE is done is the ITU which is the EU centre for the study of highly radioactive materials.
Materials in a high-radiation environment (such as a reactor) can undergo unique behaviors such as swelling
[Armin F. Lietzke (Jan 1970) Simplified Analysis of Nuclear Fuel Pin Swelling]
"The effect of fuel swelling on strains in the cladding of cylindrical fuel pins is analyzed. Simplifying assumptions are made to permit solutions for strain rates in terms of dimensionless parameters. The results of the analysis are presented in the form of equations and graphs which illustrate the volumetric swelling of the fuel and the strain rate of the fuel pin clad." and non-thermal creep. If there are nuclear reactions within the material (such as what happens in the fuel), the stoichiometry will also change slowly over time. These behaviors can lead to new material properties, cracking, and fission gas release.
The
thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
of
uranium dioxide
Uranium dioxide or uranium(IV) oxide (), also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reac ...
is low; it is affected by
porosity
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measure ...
and burn-up. The burn-up results in
fission products
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the relea ...
being dissolved in the
lattice (such as
lanthanides), the precipitation of fission products such as
palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself nam ...
, the formation of fission gas
bubbles due to fission products such as
xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
and
krypton
Krypton (from grc, κρυπτός, translit=kryptos 'the hidden one') is a chemical element with the symbol Kr and atomic number 36. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts in the atmosphere and is often ...
and radiation damage of the lattice. The low thermal conductivity can lead to overheating of the center part of the pellets during use. The porosity results in a decrease in both the thermal conductivity of the fuel and the swelling which occurs during use.
According to the
International Nuclear Safety Center
Argonne National Laboratory is a science and engineering research national laboratory operated by UChicago Argonne LLC for the United States Department of Energy. The facility is located in Lemont, Illinois, outside of Chicago, and is the larg ...
[Nuclear Engineering Division, Argonne National Laboratory, US Department of Energy (15 January 2008) International Nuclear Safety Center (INSC)]
/ref> the thermal conductivity of uranium dioxide can be predicted under different conditions by a series of equations.
The bulk density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematicall ...
of the fuel can be related to the thermal conductivity
Where ''ρ'' is the bulk density of the fuel and ''ρ''td is the theoretical density of the uranium dioxide
Uranium dioxide or uranium(IV) oxide (), also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder that naturally occurs in the mineral uraninite. It is used in nuclear fuel rods in nuclear reac ...
.
Then the thermal conductivity of the porous phase (''K''''f'') is related to the conductivity of the perfect phase (''K''''o'', no porosity) by the following equation. Note that ''s'' is a term for the shape factor of the holes.
:''K''''f'' = ''K''''o''(1 − ''p''/1 + (''s'' − 1)''p'')
Rather than measuring the thermal conductivity using the traditional methods such as Lees' disk, the Forbes' method, or Searle's bar, it is common to use Laser Flash Analysis where a small disc of fuel is placed in a furnace. After being heated to the required temperature one side of the disc is illuminated with a laser pulse, the time required for the heat wave to flow through the disc, the density of the disc, and the thickness of the disk can then be used to calculate and determine the thermal conductivity.
:''λ'' = ''ρC''''p''''α''
*''λ'' thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
*''ρ'' density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematicall ...
*''C''''p'' heat capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K).
Heat capacity ...
*''α'' thermal diffusivity
In heat transfer analysis, thermal diffusivity is the thermal conductivity divided by density and specific heat capacity at constant pressure. It measures the rate of transfer of heat of a material from the hot end to the cold end. It has the SI ...
If ''t''1/2 is defined as the time required for the non illuminated surface to experience half its final temperature rise then.
:''α'' = 0.1388 ''L''2/''t''1/2
*''L'' is the thickness of the disc
For details see K. Shinzato and T. Baba (2001).[K. Shinzato and T. Baba (2001]
''Journal of Thermal Analysis and Calorimetry'', Vol. 64 (2001) 413–422. A Laser Flash Apparatus for Thermal Diffusivity and Specific Heat Capacity Measurements
/ref>
Radioisotope decay fuels
Radioisotope battery
An atomic battery (also called a nuclear battery or radioisotope battery) is a device which uses the radioactive decay to generate electricity. These systems use radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferr ...
s that produce low energy beta particles or sometimes alpha particles of varying energies. Low energy beta particles are needed to prevent the production of high energy penetrating bremsstrahlung
''Bremsstrahlung'' (), from "to brake" and "radiation"; i.e., "braking radiation" or "deceleration radiation", is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typicall ...
radiation that would require heavy shielding. Radioisotopes such as plutonium-238
Plutonium-238 (238Pu or Pu-238) is a fissile, radionuclide, radioactive isotope of plutonium that has a half-life of 87.7 years.
Plutonium-238 is a very powerful alpha emitter; as alpha particles are easily blocked, this makes the plutonium-238 ...
, curium-242
Curium (96Cm) is an artificial element with an atomic number of 96. Because it is an artificial element, a standard atomic weight cannot be given, and it has no stable isotopes. The first isotope synthesized was 242Cm in 1944, which has 146 neutr ...
, curium-244 and strontium-90 have been used. Tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus ...
, nickel-63, promethium-147, and technetium-99 have been tested.
There are two main categories of atomic batteries: thermal and non-thermal. The non-thermal atomic batteries, which have many different designs, exploit charged alpha and beta particles. These designs include the direct charging generators, betavoltaics, the optoelectric nuclear battery, and the radioisotope piezoelectric generator. The thermal atomic batteries on the other hand, convert the heat from the radioactive decay to electricity. These designs include thermionic converter, thermophotovoltaic cells, alkali-metal thermal to electric converter, and the most common design, the radioisotope thermoelectric generator.
Radioisotope thermoelectric generator
 A
A radioisotope thermoelectric generator
A radioisotope thermoelectric generator (RTG, RITEG), sometimes referred to as a radioisotope power system (RPS), is a type of nuclear battery that uses an array of thermocouples to convert the heat released by the decay of a suitable radioacti ...
(RTG) is a simple electrical generator
In electricity generation, a generator is a device that converts motive power (mechanical energy) or fuel-based power (chemical energy) into electric power for use in an external electrical circuit, circuit. Sources of mechanical energy include s ...
which converts heat into electricity
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as describ ...
from a radioisotope using an array of thermocouple
A thermocouple, also known as a "thermoelectrical thermometer", is an electrical device consisting of two dissimilar electrical conductors forming an electrical junction. A thermocouple produces a temperature-dependent voltage as a result of th ...
s.
has become the most widely used fuel for RTGs, in the form of plutonium dioxide. It has a half-life of 87.7 years, reasonable energy density, and exceptionally low gamma and neutron radiation levels. Some Russian terrestrial RTGs have used ; this isotope has a shorter half-life and a much lower energy density, but is cheaper. Early RTGs, first built in 1958 by the U.S. Atomic Energy Commission, have used . This fuel provides phenomenally huge energy density, (a single gram of polonium-210 generates 140 watts thermal) but has limited use because of its very short half-life and gamma production, and has been phased out of use for this application.

Radioisotope heater unit (RHU)
A radioisotope heater unit
Radioisotope heater units (RHU) are small devices that provide heat through radioactive decay. They are similar to tiny radioisotope thermoelectric generators (RTG) and normally provide about one watt of heat each, derived from the decay of a fe ...
(RHU) typically provides about 1 watt
The watt (symbol: W) is the unit of power or radiant flux in the International System of Units (SI), equal to 1 joule per second or 1 kg⋅m2⋅s−3. It is used to quantify the rate of energy transfer. The watt is named after James Wa ...
of heat each, derived from the decay of a few gram
The gram (originally gramme; SI unit symbol g) is a unit of mass in the International System of Units (SI) equal to one one thousandth of a kilogram.
Originally defined as of 1795 as "the absolute weight of a volume of pure water equal to ...
s of plutonium-238. This heat is given off continuously for several decades.
Their function is to provide highly localised heating of sensitive equipment (such as electronics in outer space
Outer space, commonly shortened to space, is the expanse that exists beyond Earth and its atmosphere and between celestial bodies. Outer space is not completely empty—it is a near-perfect vacuum containing a low density of particles, pred ...
). The Cassini–Huygens
''Cassini–Huygens'' ( ), commonly called ''Cassini'', was a space-research mission by NASA, the European Space Agency (ESA), and the Italian Space Agency (ASI) to send a space probe to study the planet Saturn and its system, including its ri ...
orbiter to Saturn
Saturn is the sixth planet from the Sun and the second-largest in the Solar System, after Jupiter. It is a gas giant with an average radius of about nine and a half times that of Earth. It has only one-eighth the average density of Earth; ...
contains 82 of these units (in addition to its 3 main RTGs for power generation). The Huygens probe to Titan
Titan most often refers to:
* Titan (moon), the largest moon of Saturn
* Titans, a race of deities in Greek mythology
Titan or Titans may also refer to:
Arts and entertainment
Fictional entities
Fictional locations
* Titan in fiction, fictiona ...
contains 35 devices.
Fusion fuels
Fusion fuels are fuels to use in hypothetical Fusion power
Fusion power is a proposed form of power generation that would generate electricity by using heat from nuclear fusion reactions. In a fusion process, two lighter atomic nuclei combine to form a heavier nucleus, while releasing energy. Devices d ...
reactors. They include deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two Stable isotope ratio, stable isotopes of hydrogen (the other being Hydrogen atom, protium, or hydrogen-1). The atomic nucleus, nucleus of a deuterium ato ...
(2H) and tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus ...
(3H) as well as helium-3
Helium-3 (3He see also helion) is a light, stable isotope of helium with two protons and one neutron (the most common isotope, helium-4, having two protons and two neutrons in contrast). Other than protium (ordinary hydrogen), helium-3 is th ...
(3He). Many other elements can be fused together, but the larger electrical charge of their nuclei means that much higher temperatures are required. Only the fusion of the lightest elements is seriously considered as a future energy source. Fusion of the lightest atom, 1H hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
, as is done in the Sun and stars, has also not been considered practical on Earth. Although the energy density of fusion fuel is even higher than fission fuel, and fusion reactions sustained for a few minutes have been achieved, utilizing fusion fuel as a net energy source remains only a theoretical possibility.
First-generation fusion fuel
Deuterium and tritium are both considered first-generation fusion fuels; they are the easiest to fuse, because the electrical charge on their nuclei is the lowest of all elements. The three most commonly cited nuclear reactions that could be used to generate energy are:
:2H + 3H → n (14.07 MeV) + 4He (3.52 MeV)
:2H + 2H → n (2.45 MeV) + 3He (0.82 MeV)
:2H + 2H → p (3.02 MeV) + 3H (1.01 MeV)
Second-generation fusion fuel
Second-generation fuels require either higher confinement temperatures or longer confinement time than those required of first-generation fusion fuels, but generate fewer neutrons. Neutrons are an unwanted byproduct of fusion reactions in an energy generation context, because they are absorbed by the walls of a fusion chamber, making them radioactive. They cannot be confined by magnetic fields, because they are not electrically charged. This group consists of deuterium and helium-3. The products are all charged particles, but there may be significant side reactions leading to the production of neutrons.
:2H + 3He → p (14.68 MeV) + 4He (3.67 MeV)
Third-generation fusion fuel
Third-generation fusion fuels produce only charged particles in the primary reactions, and side reactions are relatively unimportant. Since a very small amount of neutrons is produced, there would be little induced radioactivity in the walls of the fusion chamber. This is often seen as the end goal of fusion research. 3He has the highest Maxwellian reactivity of any 3rd generation fusion fuel. However, there are no significant natural sources of this substance on Earth.
:3He + 3He → 2 p + 4He (12.86 MeV)
Another potential aneutronic fusion reaction is the proton- boron reaction:
: p + 11B → 3 4He (8.7 MeV)
Under reasonable assumptions, side reactions will result in about 0.1% of the fusion power being carried by neutrons. With 123 keV, the optimum temperature for this reaction is nearly ten times higher than that for the pure hydrogen reactions, the energy confinement must be 500 times better than that required for the D-T reaction, and the power density will be 2500 times lower than for D-T.
See also
* Fissile material
* Global Nuclear Energy Partnership
* Integrated Nuclear Fuel Cycle Information System
*Lists of nuclear disasters and radioactive incidents
These are lists of nuclear disasters and radioactive incidents.
Main lists
* List of attacks on nuclear plants
* List of Chernobyl-related articles
* List of civilian nuclear accidents
* List of civilian radiation accidents
* List of ...
*Nuclear fuel bank
A nuclear fuel bank is reserve of low enriched uranium (LEU) for countries that need a backup source of LEU to fuel their nuclear reactors. Countries that do have enrichment technology would donate enriched fuel to a "bank", from which countries ...
*Nuclear fuel cycle
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the ''front end'', which are the preparation of the fuel, steps in the ''service period'' in w ...
* Reprocessed uranium
* Uranium market
Notes
References
External links
PWR fuel
*
*
Picture showing handling of a PWR bundle
*
BWR fuel
*
CANDU fuel
*
* ttp://www.nucleartourist.com/type/candu2.htm CANDU Fuel and Reactor Specifics (Nuclear Tourist)br>Candu Fuel Rods and Bundles
TRISO fuel
* ttp://www.world-nuclear.org/sym/2003/fig-htm/labf5-h.htm GT-MHR fuel compact processbr>Description of TRISO fuel for "pebbles"
* ttp://www.ijnsweb.com/?type=subscriber&action=articleinfo&id=188 Method to calculate the temperature profile in TRISO fuel
QUADRISO fuel
Conceptual Design of QUADRISO Fuel
CERMET fuel
*
Thoria-based Cermet Nuclear Fuel: Sintered Microsphere Fabrication by Spray Drying
*
Plate type fuel
List of reactors at INL and picture of ATR core
TRIGA fuel
*
Fusion fuel
Advanced fusion fuels presentation
{{DEFAULTSORT:Nuclear Fuel
Nuclear reprocessing
Nuclear technology
Nuclear chemistry
Actinides
 Nuclear fuel is material used in nuclear power stations to produce heat to power
Nuclear fuel is material used in nuclear power stations to produce heat to power 



 Tristructural-isotropic (TRISO) fuel is a type of micro-particle fuel. A particle consists of a kernel of UOX fuel (sometimes UC or UCO), which has been coated with four layers of three isotropic materials deposited through fluidized
Tristructural-isotropic (TRISO) fuel is a type of micro-particle fuel. A particle consists of a kernel of UOX fuel (sometimes UC or UCO), which has been coated with four layers of three isotropic materials deposited through fluidized  In QUADRISO particles a burnable neutron poison ( europium oxide or
In QUADRISO particles a burnable neutron poison ( europium oxide or 
 Plate-type fuel has fallen out of favor over the years. Plate-type fuel is commonly composed of enriched uranium sandwiched between metal cladding. Plate-type fuel is used in several research reactors where a high neutron flux is desired, for uses such as material irradiation studies or isotope production, without the high temperatures seen in ceramic, cylindrical fuel. It is currently used in the Advanced Test Reactor (ATR) at
Plate-type fuel has fallen out of favor over the years. Plate-type fuel is commonly composed of enriched uranium sandwiched between metal cladding. Plate-type fuel is used in several research reactors where a high neutron flux is desired, for uses such as material irradiation studies or isotope production, without the high temperatures seen in ceramic, cylindrical fuel. It is currently used in the Advanced Test Reactor (ATR) at  A
A 