
Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from
water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
. The goal is to produce water that is fit for specific purposes. Most water is purified and disinfected for human consumption (
drinking water
Drinking water is water that is used in drink or food preparation; potable water is water that is safe to be used as drinking water. The amount of drinking water required to maintain good health varies, and depends on physical activity level, a ...
), but water purification may also be carried out for a variety of other purposes, including medical, pharmacological, chemical, and industrial applications. The history of water purification includes a wide variety of methods. The methods used include physical processes such as
filtration
Filtration is a physical separation process that separates solid matter and fluid from a mixture using a ''filter medium'' that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filter ...
,
sedimentation, and
distillation
Distillation, or classical distillation, is the process of separation process, separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distilla ...
; biological processes such as
slow sand filters
Slow sand filters are used in water purification for treating raw water to produce a potable product. They are typically deep, can be rectangular or cylindrical in cross section and are used primarily to treat surface water. The length and brea ...
or
biologically active carbon; chemical processes such as
flocculation
Flocculation, in the field of chemistry, is a process by which colloidal particles come out of suspension to sediment under the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from pr ...
and
chlorination; and the use of electromagnetic radiation such as
ultraviolet light
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
.
Water purification can reduce the concentration of particulate matter including
suspended particle
In the Outline of physical science, physical sciences, a particle (or corpuscule in older texts) is a small wikt:local, localized physical body, object which can be described by several physical property, physical or chemical property, chemical ...
s,
parasite
Parasitism is a close relationship between species, where one organism, the parasite, lives on or inside another organism, the host, causing it some harm, and is adapted structurally to this way of life. The entomologist E. O. Wilson has ...
s, bacteria,
algae
Algae (; singular alga ) is an informal term for a large and diverse group of photosynthetic eukaryotic organisms. It is a polyphyletic grouping that includes species from multiple distinct clades. Included organisms range from unicellular mic ...
, viruses, and fungi as well as reduce the concentration of a range of dissolved and particulate matter.
The standards for drinking
water quality
Water quality refers to the chemical, physical, and biological characteristics of water based on the standards of its usage. It is most frequently used by reference to a set of standards against which compliance, generally achieved through tr ...
are typically set by governments or by international standards. These standards usually include minimum and maximum concentrations of contaminants, depending on the intended use of the water.
Visual inspection which
cannot determine if water meets their quality standards. Simple procedures such as
boiling
Boiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point, the temperature at which the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. Th ...
or the use of a household
activated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that increase the surface area avail ...
filter are not sufficient for treating all possible contaminants that may be present in water from an unknown source. Even natural
spring water – considered safe for all practical purposes in the 19th century – must now be tested before determining what kind of treatment, if any, is needed.
Chemical and
microbiological analysis, while expensive, are the only way to obtain the information necessary for deciding on the appropriate method of purification.
According to a 2007
World Health Organization
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. The WHO Constitution states its main objective as "the attainment by all peoples of the highest possible level of h ...
(WHO) report, 1.1
billion people lack access to an improved drinking water supply; 88% of the 4 billion annual cases of
diarrheal disease
Diarrhea, also spelled diarrhoea, is the condition of having at least three loose, liquid, or watery bowel movements each day. It often lasts for a few days and can result in dehydration due to fluid loss. Signs of dehydration often begin wi ...
are attributed to unsafe water and inadequate
sanitation
Sanitation refers to public health conditions related to clean drinking water and treatment and disposal of human excreta and sewage. Preventing human contact with feces is part of sanitation, as is hand washing with soap. Sanitation systems ...
and hygiene, while 1.8 million people die from
diarrhoea
Diarrhea, also spelled diarrhoea, is the condition of having at least three loose, liquid, or watery bowel movements each day. It often lasts for a few days and can result in dehydration due to fluid loss. Signs of dehydration often begin wi ...
l disease each year. The WHO estimates that 94% of these diarrhoeal disease cases are preventable through modifications to the environment, including access to safe water. Simple techniques for treating water at home, such as chlorination, filters, and solar disinfection, and for storing it in safe containers could save a huge number of lives each year. Reducing deaths from
waterborne diseases is a major
public health
Public health is "the science and art of preventing disease, prolonging life and promoting health through the organized efforts and informed choices of society, organizations, public and private, communities and individuals". Analyzing the det ...
goal in developing countries.
Sources of water
#
Groundwater
Groundwater is the water present beneath Earth's surface in rock and soil pore spaces and in the fractures of rock formations. About 30 percent of all readily available freshwater in the world is groundwater. A unit of rock or an unconsolidate ...
: The water emerging from some deep ground water may have fallen as rain many tens, hundreds, or thousands of years ago.
Soil
Soil, also commonly referred to as earth or dirt, is a mixture of organic matter, minerals, gases, liquids, and organisms that together support life. Some scientific definitions distinguish ''dirt'' from ''soil'' by restricting the former te ...
and rock layers naturally filter the ground water to a high degree of clarity and often, it does not require additional treatment besides adding
chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
or
chloramines
Chloramines refer to derivatives of ammonia and organic amines wherein one or more N-H bonds have been replaced by N-Cl bonds. Two classes of compounds are considered: inorganic chloramines and organic chloramines.
Inorganic chloramines
Inorgan ...
as secondary disinfectants. Such water may emerge as springs,
artesian spring
An artesian aquifer is a confined aquifer containing groundwater under positive pressure. An artesian aquifer has trapped water, surrounded by layers of impermeable rock or clay, which apply positive pressure to the water contained within th ...
s, or may be extracted from boreholes or wells. Deep ground water is generally of very high
bacteriological quality (i.e., pathogenic bacteria or the pathogenic protozoa are typically absent), but the water may be rich in dissolved solids, especially
carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate g ...
s and
sulfates of
calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to ...
and
magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
. Depending on the
strata
In geology and related fields, a stratum ( : strata) is a layer of rock or sediment characterized by certain lithologic properties or attributes that distinguish it from adjacent layers from which it is separated by visible surfaces known as ei ...
through which the water has flowed, other ions may also be present including
chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts ...
, and
bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochemic ...
. There may be a requirement to reduce the iron or
manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy use ...
content of this water to make it acceptable for drinking, cooking, and laundry use. Primary
disinfection may also be required. Where
groundwater recharge
Groundwater recharge or deep drainage or deep percolation is a hydrologic process, where water moves downward from surface water to groundwater. Recharge is the primary method through which water enters an aquifer. This process usually occurs in ...
is practised (a process in which river water is injected into an aquifer to store the water in times of plenty so that it is available in times of drought), the groundwater may require additional treatment depending on applicable state and federal regulations.
#Upland lakes and
reservoirs: Typically located in the headwaters of river systems, upland reservoirs are usually sited above any human habitation and may be surrounded by a protective zone to restrict the opportunities for contamination. Bacteria and pathogen levels are usually low, but some bacteria,
protozoa
Protozoa (singular: protozoan or protozoon; alternative plural: protozoans) are a group of single-celled eukaryotes, either free-living or parasitic, that feed on organic matter such as other microorganisms or organic tissues and debris. Histo ...
or
algae
Algae (; singular alga ) is an informal term for a large and diverse group of photosynthetic eukaryotic organisms. It is a polyphyletic grouping that includes species from multiple distinct clades. Included organisms range from unicellular mic ...
will be present. Where uplands are forested or peaty,
humic acid Humic substances (HS) are organic compounds that are important components of humus, the major organic fraction of soil, peat, and coal (and also a constituent of many upland streams, dystrophic lakes, and ocean water). For a long era in the 19th ...
s can colour the water. Many upland sources have low
pH which require adjustment.
#Rivers,
canal
Canals or artificial waterways are waterways or engineered channels built for drainage management (e.g. flood control and irrigation) or for conveyancing water transport vehicles (e.g. water taxi). They carry free, calm surface flow un ...
s and low land reservoirs: Low land surface waters will have a significant bacterial load and may also contain algae, suspended solids and a variety of dissolved constituents.
#
Atmospheric water generation
An atmospheric water generator (AWG), is a device that extracts water from humid ambient air, producing potable water. Water vapor in the air can be extracted by multiple techniques, including condensation - cooling the air below its dew point, exp ...
is a new technology that can provide high quality drinking water by extracting water from the air by cooling the air and thus condensing water vapour.
#
Rainwater harvesting
Rainwater harvesting (RWH) is the collection and storage of rain, rather than allowing it to run off. Rainwater is collected from a roof-like surface and redirected to a tank, cistern, deep pit (well, shaft, or borehole), aquifer, or a reservoir w ...
or
fog collection which collect water from the atmosphere can be used especially in areas with significant dry seasons and in areas which experience fog even when there is little rain.
#
Desalination
Desalination is a process that takes away mineral components from saline water. More generally, desalination refers to the removal of salts and minerals from a target substance, as in Soil salinity control, soil desalination, which is an issue f ...
of
seawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has appro ...
by
distillation
Distillation, or classical distillation, is the process of separation process, separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distilla ...
or
reverse osmosis
Reverse osmosis (RO) is a water purification process that uses a partially permeable membrane to separate ions, unwanted molecules and larger particles from drinking water. In reverse osmosis, an applied pressure is used to overcome osmotic pre ...
.
#
Surface water
Surface water is water located on top of land forming terrestrial (inland) waterbodies, and may also be referred to as ''blue water'', opposed to the seawater and waterbodies like the ocean.
The vast majority of surface water is produced by prec ...
: Freshwater bodies that are open to the atmosphere and are not designated as groundwater are termed surface waters.
Treatment

Goals
The goals of the treatment are to remove unwanted constituents in the water and to make it safe to drink or fit for a specific purpose in industry or medical applications. Widely varied techniques are available to remove contaminants like fine solids, micro-organisms and some dissolved inorganic and organic materials, or
environmental persistent pharmaceutical pollutants. The choice of method will depend on the quality of the water being treated, the cost of the treatment process and the quality standards expected of the processed water.
The processes below are the ones commonly used in water purification plants. Some or most may not be used depending on the scale of the plant and quality of the raw (source) water.
Pretreatment
#Pumping and containment – The majority of water must be pumped from its source or directed into pipes or holding tanks. To avoid adding contaminants to the water, this physical infrastructure must be made from appropriate materials and constructed so that accidental contamination does not occur.
#Screening (''see also
screen filter'') – The first step in purifying surface water is to remove large debris such as sticks, leaves, rubbish and other large particles which may interfere with subsequent purification steps. Most deep groundwater does not need screening before other purification steps.
#Storage – Water from rivers may also be stored in
bankside reservoirs
A reservoir (; from French ''réservoir'' ) is an enlarged lake behind a dam. Such a dam may be either artificial, built to store fresh water or it may be a natural formation.
Reservoirs can be created in a number of ways, including contro ...
for periods between a few days and many months to allow natural biological purification to take place. This is especially important if treatment is by
slow sand filters. Storage reservoirs also provide a buffer against short periods of drought or to allow water supply to be maintained during transitory
pollution
Pollution is the introduction of contaminants into the natural environment that cause adverse change. Pollution can take the form of any substance (solid, liquid, or gas) or energy (such as radioactivity, heat, sound, or light). Pollutants, the ...
incidents in the source river.
#Pre-chlorination – In many plants the incoming water was chlorinated to minimise the growth of fouling organisms on the pipe-work and tanks. Because of the potential adverse quality effects (see chlorine below), this has largely been discontinued.
pH adjustment
Pure water has a
pH close to 7 (neither
alkaline
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a base (chemistry), basic, ionic compound, ionic salt (chemistry), salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as ...
nor
acidic).
Sea water
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approx ...
can have pH values that range from 7.5 to 8.4 (moderately alkaline). Fresh water can have widely ranging pH values depending on the geology of the
drainage basin
A drainage basin is an area of land where all flowing surface water converges to a single point, such as a river mouth, or flows into another body of water, such as a lake or ocean. A basin is separated from adjacent basins by a perimeter, t ...
or
aquifer
An aquifer is an underground layer of water-bearing, permeable rock, rock fractures, or unconsolidated materials (gravel, sand, or silt). Groundwater from aquifers can be extracted using a water well. Aquifers vary greatly in their characterist ...
and the influence of contaminant inputs (
acid rain
Acid rain is rain or any other form of precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists between 6.5 and 8.5, but acid ...
). If the water is acidic (lower than 7),
lime
Lime commonly refers to:
* Lime (fruit), a green citrus fruit
* Lime (material), inorganic materials containing calcium, usually calcium oxide or calcium hydroxide
* Lime (color), a color between yellow and green
Lime may also refer to:
Botany ...
,
soda ash, or
sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
can be added to raise the pH during water purification processes. Lime addition increases the calcium ion concentration, thus raising the water hardness. For highly acidic waters, forced draft
degasifiers can be an effective way to raise the pH, by stripping dissolved carbon dioxide from the water. Making the water alkaline helps
coagulation
Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism o ...
and
flocculation
Flocculation, in the field of chemistry, is a process by which colloidal particles come out of suspension to sediment under the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from pr ...
processes work effectively and also helps to minimise the risk of lead being dissolved from lead pipes and from lead
solder in pipe fittings. Sufficient alkalinity also reduces the corrosiveness of water to iron pipes. Acid (
carbonic acid,
hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
or
sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
) may be added to alkaline waters in some circumstances to lower the pH. Alkaline water (above pH 7.0) does not necessarily mean that lead or copper from the plumbing system will not be dissolved into the water. The ability of water to precipitate calcium carbonate to protect metal surfaces and reduce the likelihood of toxic metals being dissolved in water is a function of pH, mineral content, temperature, alkalinity and calcium concentration.
Coagulation and flocculation

One of the first steps in most conventional water purification processes is the addition of chemicals to assist in the removal of particles suspended in water. Particles can be inorganic such as
clay
Clay is a type of fine-grained natural soil material containing clay minerals (hydrous aluminium phyllosilicates, e.g. kaolin, Al2 Si2 O5( OH)4).
Clays develop plasticity when wet, due to a molecular film of water surrounding the clay par ...
and
silt
Silt is granular material of a size between sand and clay and composed mostly of broken grains of quartz. Silt may occur as a soil (often mixed with sand or clay) or as sediment mixed in suspension with water. Silt usually has a floury feel when ...
or organic such as
algae
Algae (; singular alga ) is an informal term for a large and diverse group of photosynthetic eukaryotic organisms. It is a polyphyletic grouping that includes species from multiple distinct clades. Included organisms range from unicellular mic ...
, bacteria,
viruses
A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea.
Since Dmitri Ivanovsky's 1 ...
,
protozoa
Protozoa (singular: protozoan or protozoon; alternative plural: protozoans) are a group of single-celled eukaryotes, either free-living or parasitic, that feed on organic matter such as other microorganisms or organic tissues and debris. Histo ...
and
natural organic matter
Organic matter, organic material, or natural organic matter refers to the large source of carbon-based compounds found within natural and engineered, terrestrial, and aquatic environments. It is matter composed of organic compounds that have c ...
. Inorganic and organic particles contribute to the
turbidity
Turbidity is the cloudiness or haziness of a fluid caused by large numbers of individual particles that are generally invisible to the naked eye, similar to smoke in air. The measurement of turbidity is a key test of water quality.
Fluids can ...
and color of water.
The addition of inorganic coagulants such as
aluminium sulfate
Aluminium sulfate is a salt with the chemical formula, formula aluminium, Al2sulfate, (SO4)3. It is soluble in water and is mainly used as a Coagulation (water treatment), coagulating agent (promoting particle collision by neutralizing charge) in ...
(or
alum
An alum () is a type of chemical compound, usually a hydrated double salt, double sulfate salt (chemistry), salt of aluminium with the general chemical formula, formula , where is a valence (chemistry), monovalent cation such as potassium or a ...
) or iron (III) salts such as
iron(III) chloride
Iron(III) chloride is the inorganic compound with the formula . Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The col ...
cause several simultaneous chemical and physical interactions on and among the particles. Within seconds, negative charges on the particles are neutralised by inorganic coagulants. Also within seconds, metal hydroxide precipitates of the iron and aluminium ions begin to form. These precipitates combine into larger particles under natural processes such as
Brownian motion and through induced mixing which is sometimes referred to as
flocculation
Flocculation, in the field of chemistry, is a process by which colloidal particles come out of suspension to sediment under the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from pr ...
. Amorphous metal hydroxides are known as "floc". Large, amorphous aluminium and iron (III) hydroxides adsorb and enmesh particles in suspension and facilitate the removal of particles by subsequent processes of
sedimentation and
filtration
Filtration is a physical separation process that separates solid matter and fluid from a mixture using a ''filter medium'' that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filter ...
.
[Edzwald, James K., ed. (2011). ''Water Quality and Treatment''. 6th Edition. New York:McGraw-Hill.https://www.accessengineeringlibrary.com/content/book/9780071630115?implicit-login=true ]
Aluminum hydroxides are formed within a fairly narrow pH range, typically: 5.5 to about 7.7. Iron (III) hydroxides can form over a larger pH range including pH levels lower than are effective for alum, typically: 5.0 to 8.5.
[Crittenden, John C., et al., eds. (2005). ''Water Treatment: Principles and Design.'' 2nd Edition. Hoboken, NJ:Wiley. ]
In the literature, there is much debate and confusion over the usage of the terms coagulation and flocculation: Where does coagulation end and flocculation begin? In water purification plants, there is usually a high energy, rapid mix unit process (detention time in seconds) whereby the coagulant chemicals are added followed by flocculation basins (detention times range from 15 to 45 minutes) where low energy inputs turn large paddles or other gentle mixing devices to enhance the formation of floc. In fact, coagulation and flocculation processes are ongoing once the metal salt coagulants are added.
Organic polymers were developed in the 1960s as aids to coagulants and, in some cases, as replacements for the inorganic metal salt coagulants. Synthetic organic polymers are high molecular weight compounds that carry negative, positive or neutral charges. When organic polymers are added to water with particulates, the high molecular weight compounds adsorb onto particle surfaces and through interparticle bridging coalesce with other particles to form floc.
PolyDADMAC is a popular cationic (positively charged) organic polymer used in water purification plants.
[
]
Sedimentation
Waters exiting the flocculation basin may enter the sedimentation basin, also called a clarifier or settling basin. It is a large tank with low water velocities, allowing floc to settle to the bottom. The sedimentation basin is best located close to the flocculation basin so the transit between the two processes does not permit settlement or floc break up. Sedimentation basins may be rectangular, where water flows from end to end, or circular where flow is from the centre outward. Sedimentation basin outflow is typically over a weir so only a thin top layer of water—that furthest from the sludge—exits.
In 1904, Allen Hazen
Allen Hazen (August 28, 1869 – July 26, 1930) was an expert in hydraulics, flood control, water purification and sewage treatment. His career extended from 1888 to 1930 and he is, perhaps, best known for his contributions to hydraulics with the ...
showed that the efficiency of a sedimentation process was a function of the particle settling velocity, the flow through the tank and the surface area of tank. Sedimentation tanks are typically designed within a range of overflow rates of 0.5 to 1.0 gallons per minute per square foot (or 1250 to 2500 litres per square meter per hour). In general, sedimentation basin efficiency is not a function of detention time or depth of the basin. Although, basin depth must be sufficient so that water currents do not disturb the sludge and settled particle interactions are promoted. As particle concentrations in the settled water increase near the sludge surface on the bottom of the tank, settling velocities can increase due to collisions and agglomeration of particles. Typical detention times for sedimentation vary from 1.5 to 4 hours and basin depths vary from 10 to 15 feet (3 to 4.5 meters).[
Inclined flat plates or tubes can be added to traditional sedimentation basins to improve particle removal performance. Inclined plates and tubes drastically increase the surface area available for particles to be removed in concert with Hazen's original theory. The amount of ground surface area occupied by a sedimentation basin with inclined plates or tubes can be far smaller than a conventional sedimentation basin.
]
Sludge storage and removal
As particles settle to the bottom of a sedimentation basin, a layer of sludge is formed on the floor of the tank which must be removed and treated. The amount of sludge generated is significant, often 3 to 5 per cent of the total volume of water to be treated. The cost of treating and disposing of the sludge can impact the operating cost of a water treatment plant. The sedimentation basin may be equipped with mechanical cleaning devices that continually clean its bottom, or the basin can be periodically taken out of service and cleaned manually.
Floc blanket clarifiers
A subcategory of sedimentation is the removal of particulates by entrapment in a layer of suspended floc as the water is forced upward. The major advantage of floc blanket clarifiers is that they occupy a smaller footprint than conventional sedimentation. The disadvantages are that particle removal efficiency can be highly variable depending on changes in influent water quality and influent water flow rate.[
]
Dissolved air flotation
When particles to be removed do not settle out of solution easily, dissolved air flotation (DAF) is often used. After coagulation and flocculation processes, water flows to DAF tanks where air diffusers on the tank bottom create fine bubbles that attach to the floc resulting in a floating mass of concentrated floc. The floating floc blanket is removed from the surface and clarified water is withdrawn from the bottom of the DAF tank.
Water supplies that are particularly vulnerable to unicellular algae blooms and supplies with low turbidity and high colour often employ DAF.
Filtration
After separating most floc, the water is filtered as the final step to remove remaining suspended particles and unsettled floc.
Rapid sand filters
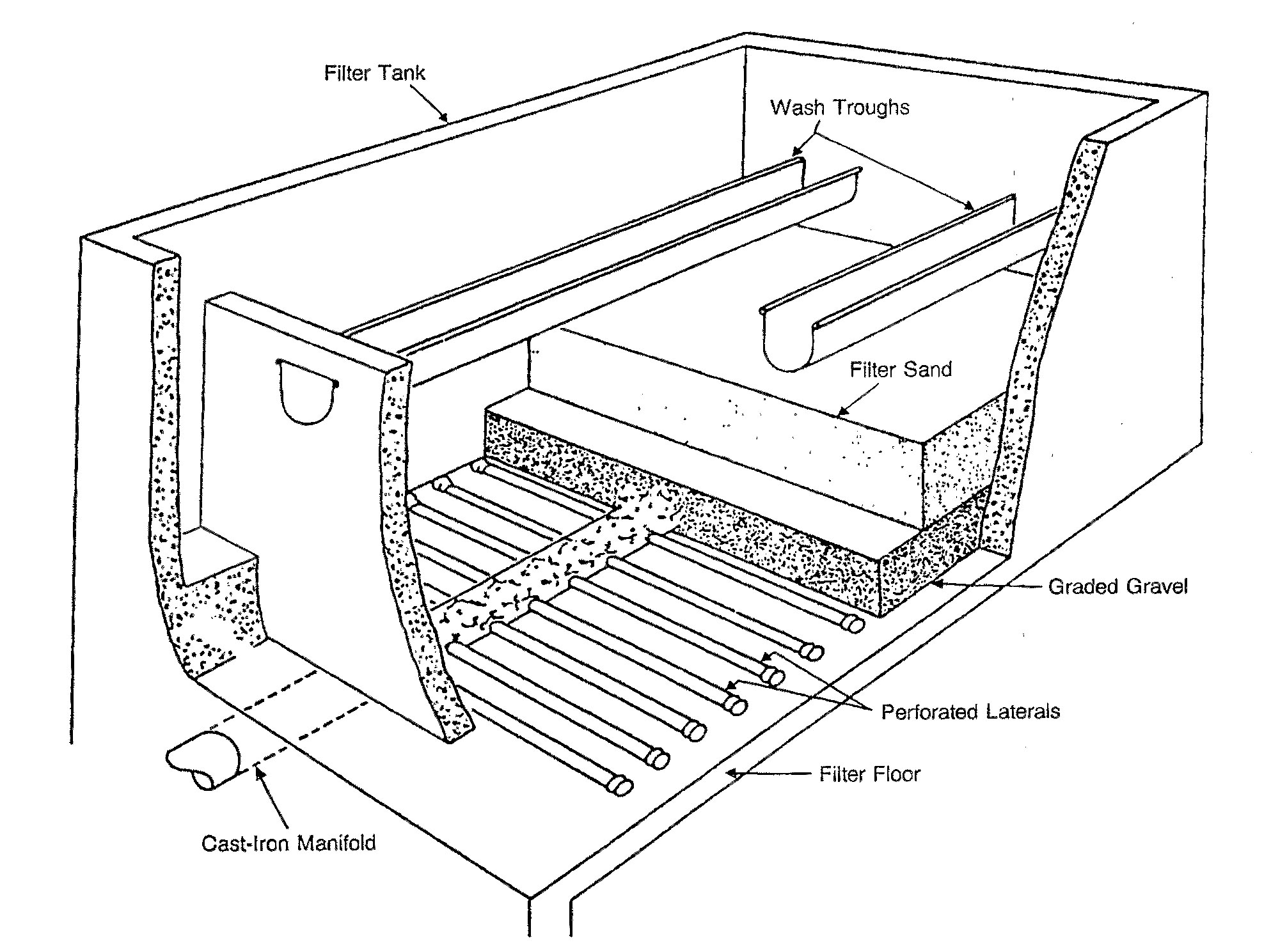 The most common type of filter is a
The most common type of filter is a rapid sand filter
The rapid sand filter or rapid gravity filter is a type of filter used in water purification and is commonly used in municipal drinking water facilities as part of a multiple-stage treatment system.
Rapid sand filters were first developed in the ...
. Water moves vertically through sand which often has a layer of activated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that increase the surface area avail ...
or anthracite coal
Anthracite, also known as hard coal, and black coal, is a hard, compact variety of coal that has a submetallic luster. It has the highest carbon content, the fewest impurities, and the highest energy density of all types of coal and is the high ...
above the sand. The top layer removes organic compounds, which contribute to taste and odour. The space between sand particles is larger than the smallest suspended particles, so simple filtration is not enough. Most particles pass through surface layers but are trapped in pore spaces or adhere to sand particles. Effective filtration extends into the depth of the filter. This property of the filter is key to its operation: if the top layer of sand were to block all the particles, the filter would quickly clog.[United States Environmental Protection Agency (EPA)(1990). Cincinnati, OH]
"Technologies for Upgrading Existing or Designing New Drinking Water Treatment Facilities."
Document no. EPA/625/4-89/023.
To clean the filter, water is passed quickly upward through the filter, opposite the normal direction (called ''backflushing'' or '' backwashing'') to remove embedded or unwanted particles. Prior to this step, compressed air may be blown up through the bottom of the filter to break up the compacted filter media to aid the backwashing process; this is known as ''air scouring''. This contaminated water can be disposed of, along with the sludge from the sedimentation basin, or it can be recycled by mixing with the raw water entering the plant although this is often considered poor practice since it re-introduces an elevated concentration of bacteria into the raw water.
Some water treatment plants employ pressure filters. These work on the same principle as rapid gravity filters, differing in that the filter medium is enclosed in a steel vessel and the water is forced through it under pressure.
Advantages:
* Filters out much smaller particles than paper and sand filters can.
* Filters out virtually all particles larger than their specified pore sizes.
* They are quite thin and so liquids flow through them fairly rapidly.
* They are reasonably strong and so can withstand pressure differences across them of typically 2–5 atmospheres.
* They can be cleaned (back flushed) and reused.
Slow sand filters
Slow sand filters may be used where there is sufficient land and space, as the water flows very slowly through the filters. These filters rely on biological treatment processes for their action rather than physical filtration. They are carefully constructed using graded layers of sand, with the coarsest sand, along with some gravel, at the bottom and the finest sand at the top. Drains at the base convey treated water away for disinfection. Filtration depends on the development of a thin biological layer, called the zoogleal layer or Schmutzdecke, on the surface of the filter. An effective slow sand filter may remain in service for many weeks or even months, if the pretreatment is well designed, and produces water with a very low available nutrient level which physical methods of treatment rarely achieve. Very low nutrient levels allow water to be safely sent through distribution systems with very low disinfectant levels, thereby reducing consumer irritation over offensive levels of chlorine and chlorine by-products. Slow sand filters are not backwashed; they are maintained by having the top layer of sand scraped off when the flow is eventually obstructed by biological growth.
Bank filtration
In bank filtration
River Bank filtration is a type of filtration that works by passing water to be purified for use as drinking water through the banks of a river or lake. It is then drawn off by extraction wells some distance away from the water body. The process ...
, natural sediments in a riverbank are used to provide the first stage of contaminant filtration. While typically not clean enough to be used directly for drinking water, the water gained from the associated extraction wells is much less problematic than river water taken directly from the river.
Membrane filtration
Membrane filters are widely used for filtering both drinking water and sewage
Sewage (or domestic sewage, domestic wastewater, municipal wastewater) is a type of wastewater that is produced by a community of people. It is typically transported through a sewer system. Sewage consists of wastewater discharged from residenc ...
. For drinking water, membrane filters can remove virtually all particles larger than 0.2 μm—including ''giardia
''Giardia'' ( or ) is a genus of anaerobic flagellated protozoan parasites of the phylum Metamonada that colonise and reproduce in the small intestines of several vertebrates, causing the disease giardiasis. Their life cycle alternates between ...
'' and ''cryptosporidium
''Cryptosporidium'', sometimes informally called crypto, is a genus of apicomplexan parasitic alveolates that can cause a respiratory and gastrointestinal illness (cryptosporidiosis) that primarily involves watery diarrhea (intestinal cryptosp ...
.'' Membrane filters are an effective form of tertiary treatment when it is desired to reuse the water for industry, for limited domestic purposes, or before discharging the water into a river that is used by towns further downstream. They are widely used in industry, particularly for beverage preparation (including bottled water). However no filtration can remove substances that are actually dissolved in the water such as phosphates, nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
s and heavy metal ions.
Removal of ions and other dissolved substances
Ultrafiltration membranes
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Bi ...
use polymer membranes with chemically formed microscopic pores that can be used to filter out dissolved substances avoiding the use of coagulants. The type of membrane media determines how much pressure is needed to drive the water through and what sizes of micro-organisms can be filtered out.
Ion exchange:zeolite
Zeolites are microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a metal ion or H+. These pos ...
-packed columns to replace unwanted ions. The most common case is water softening
Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions. Soft water also extend ...
consisting of removal of Ca2+ and Mg2+ ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s replacing them with benign (soap friendly) Na+ or K+ ions. Ion-exchange resins are also used to remove toxic ions such as nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
, lead, mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
, arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but ...
and many others.
Precipitative softening:hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to localized plastic deformation induced by either mechanical indentation or abrasion. In general, different materials differ in their hardness; for example hard ...
(calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to ...
and magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
ions) is treated with lime (calcium oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, Caustic (substance), caustic, alkaline, crystalline solid at room temperature. The broadly used term "''lime (material), lime''" co ...
) and/or soda-ash (sodium carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
) to precipitate calcium carbonate out of solution utilising the common-ion effect.
Electrodeionization
Electrodeionization (EDI) is a water treatment technology that utilizes electricity, ion exchange membranes, and resin to deionize water and separate dissolved ions (impurities) from it. It differs from other water purification technologies in ...
:electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials de ...
and a negative electrode. Ion-exchange membranes
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Bi ...
allow only positive ions to migrate from the treated water toward the negative electrode and only negative ions toward the positive electrode. High purity deionised water is produced continuously, similar to ion-exchange treatment. Complete removal of ions from water is possible if the right conditions are met. The water is normally pre-treated with a reverse osmosis
Reverse osmosis (RO) is a water purification process that uses a partially permeable membrane to separate ions, unwanted molecules and larger particles from drinking water. In reverse osmosis, an applied pressure is used to overcome osmotic pre ...
unit to remove non-ionic organic contaminants
Organic may refer to:
* Organic, of or relating to an organism, a living entity
* Organic, of or relating to an anatomical organ
Chemistry
* Organic matter, matter that has come from a once-living organism, is capable of decay or is the product o ...
, and with gas transfer membranes to remove carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
. A water recovery of 99% is possible if the concentrate stream is fed to the RO inlet.
Disinfection
 Disinfection is accomplished both by filtering out harmful micro-organisms and by adding disinfectant chemicals. Water is disinfected to kill any pathogens which pass through the filters and to provide a residual dose of disinfectant to kill or inactivate potentially harmful micro-organisms in the storage and distribution systems. Possible pathogens include
Disinfection is accomplished both by filtering out harmful micro-organisms and by adding disinfectant chemicals. Water is disinfected to kill any pathogens which pass through the filters and to provide a residual dose of disinfectant to kill or inactivate potentially harmful micro-organisms in the storage and distribution systems. Possible pathogens include viruses
A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea.
Since Dmitri Ivanovsky's 1 ...
, bacteria, including ''Salmonella
''Salmonella'' is a genus of rod-shaped (bacillus) Gram-negative bacteria of the family Enterobacteriaceae. The two species of ''Salmonella'' are ''Salmonella enterica'' and ''Salmonella bongori''. ''S. enterica'' is the type species and is fur ...
'', ''Cholera
Cholera is an infection of the small intestine by some strains of the bacterium ''Vibrio cholerae''. Symptoms may range from none, to mild, to severe. The classic symptom is large amounts of watery diarrhea that lasts a few days. Vomiting and ...
'', ''Campylobacter
''Campylobacter'' (meaning "curved bacteria") is a genus of Gram-negative bacteria. ''Campylobacter'' typically appear comma- or s-shaped, and are motile. Some ''Campylobacter'' species can infect humans, sometimes causing campylobacteriosis, a d ...
'' and ''Shigella
''Shigella'' is a genus of bacteria that is Gram-negative, facultative anaerobic, non-spore-forming, nonmotile, rod-shaped, and genetically closely related to ''E. coli''. The genus is named after Kiyoshi Shiga, who first discovered it in 1897. ...
'', and protozoa
Protozoa (singular: protozoan or protozoon; alternative plural: protozoans) are a group of single-celled eukaryotes, either free-living or parasitic, that feed on organic matter such as other microorganisms or organic tissues and debris. Histo ...
, including '' Giardia lamblia'' and other ''cryptosporidia
Cryptosporidiosis, sometimes informally called crypto, is a parasitic disease caused by '' Cryptosporidium'', a genus of protozoan parasites in the phylum Apicomplexa. It affects the distal small intestine and can affect the respiratory tract ...
''. After the introduction of any chemical disinfecting agent, the water is usually held in temporary storage – often called a contact tank or clear well
A clear well (sometimes spelled as "clearwell") is a component of a municipal drinking water purification system. It refers to the final storage stage in the system, following the filtration and disinfection stages. The filtered water is held ...
– to allow the disinfecting action to complete.
Chlorine disinfection
The most common disinfection method involves some form of chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
or its compounds such as chloramine
Chloramines refer to derivatives of ammonia and organic amines wherein one or more N-H bonds have been replaced by N-Cl bonds. Two classes of compounds are considered: inorganic chloramines and organic chloramines.
Inorganic chloramines
Inorgan ...
or chlorine dioxide. Chlorine is a strong oxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxid ...
that rapidly kills many harmful micro-organisms. Because chlorine is a toxic gas, there is a danger of a release associated with its use. This problem is avoided by the use of sodium hypochlorite, which is a relatively inexpensive solution used in household bleach that releases free chlorine when dissolved in water. Chlorine solutions can be generated on site by electrolyzing common salt solutions. A solid form, calcium hypochlorite
Calcium hypochlorite is an inorganic compound with formula Ca(OCl)2. It is the main active ingredient of commercial products called bleaching powder, chlorine powder, or chlorinated lime, used for water treatment and as a bleaching agent. Thi ...
, releases chlorine on contact with water. Handling the solid, however, requires more routine human contact through opening bags and pouring than the use of gas cylinders or bleach, which are more easily automated. The generation of liquid sodium hypochlorite is inexpensive and also safer than the use of gas or solid chlorine. Chlorine levels up to 4 milligrams per litre (4 parts per million) are considered safe in drinking water.
All forms of chlorine are widely used, despite their respective drawbacks. One drawback is that chlorine from any source reacts with natural organic compounds in the water to form potentially harmful chemical by-products. These by-products, trihalomethanes (THMs) and haloacetic acid Haloacetic acids are carboxylic acids in which a halogen atom takes the place of a hydrogen atom in acetic acid. Thus, in a monohaloacetic acid, a single halogen would replace a hydrogen atom. For example, chloroacetic acid would have the struc ...
s (HAAs), are both carcinogenic
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substan ...
in large quantities and are regulated by the United States Environmental Protection Agency
The Environmental Protection Agency (EPA) is an independent executive agency of the United States federal government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it be ...
(EPA) and the Drinking Water Inspectorate The Drinking Water Inspectorate (DWI) is a section of Department for Environment, Food and Rural Affairs (DEFRA) set up to regulate the public water supply companies in England and Wales.
Based in Whitehall, it produces an annual report showing the ...
in the UK. The formation of THMs and haloacetic acids may be minimised by the effective removal of as many organics from the water as possible prior to chlorine addition. Although chlorine is effective in killing bacteria, it has limited effectiveness against pathogenic protozoa that form cysts in water such as '' Giardia lamblia'' and ''Cryptosporidium
''Cryptosporidium'', sometimes informally called crypto, is a genus of apicomplexan parasitic alveolates that can cause a respiratory and gastrointestinal illness (cryptosporidiosis) that primarily involves watery diarrhea (intestinal cryptosp ...
''.
Chlorine dioxide disinfection
Chlorine dioxide is a faster-acting disinfectant than elemental chlorine. It is relatively rarely used because in some circumstances it may create excessive amounts of chlorite
The chlorite ion, or chlorine dioxide anion, is the halite with the chemical formula of . A chlorite (compound) is a compound that contains this group, with chlorine in the oxidation state of +3. Chlorites are also known as salts of chlorous ac ...
, which is a by-product regulated to low allowable levels in the United States. Chlorine dioxide can be supplied as an aqueous solution and added to water to avoid gas handling problems; chlorine dioxide gas accumulations may spontaneously detonate.
Chloramination
The use of chloramine
Chloramines refer to derivatives of ammonia and organic amines wherein one or more N-H bonds have been replaced by N-Cl bonds. Two classes of compounds are considered: inorganic chloramines and organic chloramines.
Inorganic chloramines
Inorgan ...
is becoming more common as a disinfectant. Although chloramine is not as strong an oxidant, it provides a longer-lasting residual than free chlorine because of its lower redox potential compared to free chlorine. It also does not readily form THMs or haloacetic acids (disinfection byproducts
Disinfection by-products (DBPs) result from chemical reactions between organic and inorganic matter in water with chemical treatment agents during the water disinfection process.
Chlorination disinfection byproducts
Chlorinated disinfection agen ...
).
It is possible to convert chlorine to chloramine by adding ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
to the water after adding chlorine. The chlorine and ammonia react to form chloramine. Water distribution systems disinfected with chloramines may experience nitrification
''Nitrification'' is the biological oxidation of ammonia to nitrite followed by the oxidation of the nitrite to nitrate occurring through separate organisms or direct ammonia oxidation to nitrate in comammox bacteria. The transformation of amm ...
, as ammonia is a nutrient for bacterial growth, with nitrates being generated as a by-product.
Ozone disinfection
Ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
is an unstable molecule which readily gives up one atom of oxygen providing a powerful oxidising agent which is toxic to most waterborne organisms. It is a very strong, broad spectrum disinfectant that is widely used in Europe and in a few municipalities in the United States and Canada. Ozone disinfection, or ozonation, is an effective method to inactivate harmful protozoa that form cysts. It also works well against almost all other pathogens. Ozone is made by passing oxygen through ultraviolet light or a "cold" electrical discharge. To use ozone as a disinfectant, it must be created on-site and added to the water by bubble contact. Some of the advantages of ozone include the production of fewer dangerous by-products and the absence of taste and odour problems (in comparison to chlorination). No residual ozone is left in the water.[Neumann, H. (1981). "Bacteriological safety of hot tap water in developing countries." ''Public Health'' Rep.84:812–814.] In the absence of a residual disinfectant in the water, chlorine or chloramine may be added throughout a distribution system to remove any potential pathogens in the distribution piping.
Ozone has been used in drinking water plants since 1906 where the first industrial ozonation plant was built in Nice
Nice ( , ; Niçard: , classical norm, or , nonstandard, ; it, Nizza ; lij, Nissa; grc, Νίκαια; la, Nicaea) is the prefecture of the Alpes-Maritimes department in France. The Nice agglomeration extends far beyond the administrative c ...
, France. The U.S. Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food s ...
has accepted ozone as being safe; and it is applied as an anti-microbiological agent for the treatment, storage, and processing of foods. However, although fewer by-products are formed by ozonation, it has been discovered that ozone reacts with bromide ions in water to produce concentrations of the suspected carcinogen bromate. Bromide can be found in fresh water supplies in sufficient concentrations to produce (after ozonation) more than 10 parts per billion (ppb) of bromatethe maximum contaminant level established by the USEPA. Ozone disinfection is also energy intensive.
Ultraviolet disinfection

Ultraviolet light
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
(UV) is very effective at inactivating cysts, in low turbidity water. UV light's disinfection effectiveness decreases as turbidity increases, a result of the absorption
Absorption may refer to:
Chemistry and biology
* Absorption (biology), digestion
**Absorption (small intestine)
*Absorption (chemistry), diffusion of particles of gas or liquid into liquid or solid materials
*Absorption (skin), a route by which ...
, scattering
Scattering is a term used in physics to describe a wide range of physical processes where moving particles or radiation of some form, such as light or sound, are forced to deviate from a straight trajectory by localized non-uniformities (including ...
, and shadowing caused by the suspended solids. The main disadvantage to the use of UV radiation is that, like ozone treatment, it leaves no residual disinfectant in the water; therefore, it is sometimes necessary to add a residual disinfectant after the primary disinfection process. This is often done through the addition of chloramines, discussed above as a primary disinfectant. When used in this manner, chloramines provide an effective residual disinfectant with very few of the negative effects of chlorination.
Over 2 million people in 28 developing countries use Solar Disinfection for daily drinking water treatment.
Ionizing radiation
Like UV, ionizing radiation
Ionizing radiation (or ionising radiation), including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel ...
(X-rays, gamma rays, and electron beams) has been used to sterilise water.
Bromination and iodinisation
Bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simila ...
and iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
can also be used as disinfectants. However, chlorine in water is over three times more effective as a disinfectant against ''Escherichia coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escher ...
'' than an equivalent concentration of bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simila ...
, and over six times more effective than an equivalent concentration of iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
. Iodine is commonly used for portable water purification
Portable water purification devices are self-contained, easily transported units used to purify water from untreated sources (such as rivers, lakes, and wells) for drinking purposes. Their main function is to eliminate pathogens, and often als ...
, and bromine is common as a swimming pool disinfectant.
Portable water purification
Portable water purification devices and methods are available for disinfection and treatment in emergencies or in remote locations. Disinfection is the primary goal, since aesthetic considerations such as taste, odour, appearance, and trace chemical contamination do not affect the short-term safety of drinking water.
Additional treatment options
# Water fluoridation: in many areas fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typ ...
is added to water with the goal of preventing tooth decay. Fluoride is usually added after the disinfection process. In the U.S., fluoridation is usually accomplished by the addition of hexafluorosilicic acid, which decomposes in water, yielding fluoride ions.
#Water conditioning: This is a method of reducing the effects of hard water. In water systems subject to heating hardness salts can be deposited as the decomposition of bicarbonate ions creates carbonate ions that precipitate out of solution. Water with high concentrations of hardness salts can be treated with soda ash (sodium carbonate) which precipitates out the excess salts, through the common-ion effect, producing calcium carbonate of very high purity. The precipitated calcium carbonate is traditionally sold to the manufacturers of toothpaste
Toothpaste is a paste or gel dentifrice used with a toothbrush to clean and maintain the aesthetics and health of teeth. Toothpaste is used to promote oral hygiene: it is an abrasive that aids in removing dental plaque and food from the teeth, a ...
. Several other methods of industrial and residential water treatment are claimed (without general scientific acceptance) to include the use of magnetic and/or electrical fields reducing the effects of hard water.
#Plumbosolvency
Plumbosolvency is the ability of a solvent, notably water, to dissolve lead. In the public supply of water this is an undesirable property. In (usually older) consumers' premises plumbosolvent water can attack lead pipes, lead service lines, and a ...
reduction: In areas with naturally acidic waters of low conductivity (i.e. surface rainfall in upland mountains of igneous
Igneous rock (derived from the Latin word ''ignis'' meaning fire), or magmatic rock, is one of the three main rock types, the others being sedimentary and metamorphic. Igneous rock is formed through the cooling and solidification of magma or ...
rocks), the water may be capable of dissolving lead from any lead pipes that it is carried in. The addition of small quantities of phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phospho ...
ion and increasing the pH slightly both assist in greatly reducing plumbo-solvency by creating insoluble lead salts on the inner surfaces of the pipes.
#Radium Removal: Some groundwater sources contain radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rather t ...
, a radioactive chemical element. Typical sources include many groundwater sources north of the Illinois River
The Illinois River ( mia, Inoka Siipiiwi) is a principal tributary of the Mississippi River and is approximately long. Located in the U.S. state of Illinois, it has a drainage basin of . The Illinois River begins at the confluence of the D ...
in Illinois
Illinois ( ) is a U.S. state, state in the Midwestern United States, Midwestern United States. Its largest metropolitan areas include the Chicago metropolitan area, and the Metro East section, of Greater St. Louis. Other smaller metropolita ...
, United States of America. Radium can be removed by ion exchange, or by water conditioning. The back flush or sludge that is produced is, however, a low-level radioactive waste
Radioactive waste is a type of hazardous waste that contains radioactive material. Radioactive waste is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, rare-earth mining, and nuclear weapons r ...
.
#Fluoride Removal: Although fluoride is added to water in many areas, some areas of the world have excessive levels of natural fluoride in the source water. Excessive levels can be toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ...
or cause undesirable cosmetic effects such as staining of teeth. Methods of reducing fluoride levels is through treatment with activated alumina
Activated alumina is manufactured from aluminium hydroxide by dehydroxylating it in a way that produces a highly porous material; this material can have a surface area significantly over 200 m²/g. The compound is used as a desiccant (to keep th ...
and bone char filter media.
Other water purification techniques
Other popular methods for purifying water, especially for local private supplies are listed below. In some countries some of these methods are used for large scale municipal supplies. Particularly important are distillation (desalination
Desalination is a process that takes away mineral components from saline water. More generally, desalination refers to the removal of salts and minerals from a target substance, as in Soil salinity control, soil desalination, which is an issue f ...
of seawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has appro ...
) and reverse osmosis.
Thermal
Bringing water to its boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
(about 100 °C or 212 F at sea level), is the oldest and most effective way since it eliminates most microbes causing intestinal
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the digestive system that leads from the mouth to the anus. The GI tract contains all the major organs of the digestive system, in humans ...
disease,[Curtis, Rick (1998]
OA Guide to Water Purification, The Backpacker's Field Manual
Random House. For human health, complete sterilisation of water is not required, since heat resistant microbes do not affect intestines.bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochemic ...
ions, resulting in partial precipitation as calcium carbonate. This is the "fur" that builds up on kettle elements, etc., in hard water areas. With the exception of calcium, boiling does not remove solutes of higher boiling point than water and in fact increases their concentration (due to some water being lost as vapour). Boiling does not leave a residual disinfectant in the water. Therefore, water that is boiled and then stored for any length of time may acquire new pathogens.
Adsorption
Granular activated carbon is a form of activated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that increase the surface area avail ...
with a high surface area. It adsorbs many compounds including many toxic compounds. Water passing through activated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that increase the surface area avail ...
is commonly used in municipal regions with organic contamination, taste or odors. Many household water filters and fish tanks use activated carbon filters to purify water. Household filters for drinking water sometimes contain silver as metallic silver nanoparticle. If water is held in the carbon block for longer periods, microorganisms can grow inside which results in fouling and contamination. Silver nanoparticles are excellent anti-bacterial material and can decompose toxic halo-organic compounds such as pesticides into non-toxic organic products. Filtered water must be used soon after it is filtered, as the low amount of remaining microbes may proliferate over time. In general, these home filters remove over 90% of the chlorine in a glass of treated water. These filters must be periodically replaced otherwise the bacterial content of the water may actually increase due to the growth of bacteria within the filter unit.
Distillation
Distillation
Distillation, or classical distillation, is the process of separation process, separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distilla ...
involves boiling water to produce water vapour. The vapour contacts a cool surface where it condenses as a liquid. Because the solutes are not normally vaporised, they remain in the boiling solution. Even distillation does not completely purify water, because of contaminants with similar boiling points and droplets of unvapourised liquid carried with the steam. However, 99.9% pure water can be obtained by distillation.
Direct contact membrane distillation (DCMD) passes heated seawater along the surface of a hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, th ...
polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
membrane. Evaporated water passes from the hot side through pores in the membrane forming a stream of cold pure water on the other side. The difference in vapour pressure between the hot and cold side helps to push water molecules through.
Reverse osmosis
Reverse osmosis
Reverse osmosis (RO) is a water purification process that uses a partially permeable membrane to separate ions, unwanted molecules and larger particles from drinking water. In reverse osmosis, an applied pressure is used to overcome osmotic pre ...
involves mechanical pressure applied to force water through a semi-permeable membrane. Contaminants are left on the other side of the membrane. Reverse osmosis is theoretically the most thorough method of large scale water purification available, although perfect semi-permeable membranes are difficult to create. Unless membranes are well-maintained, algae
Algae (; singular alga ) is an informal term for a large and diverse group of photosynthetic eukaryotic organisms. It is a polyphyletic grouping that includes species from multiple distinct clades. Included organisms range from unicellular mic ...
and other life forms can colonise the membranes.
Crystallization
Carbon dioxide or other low molecular weight gas can be mixed with contaminated water at high pressure and low temperature to exothermically form gas hydrate crystals. Hydrate may be separated by centrifuge or sedimentation. Water can be released from the hydrate crystals by heating.
''In situ'' oxidation
''In situ'' chemical oxidation (ISCO) is an advanced oxidation process. It is used for soil and/or groundwater remediation to reduce the concentrations of targeted contaminants. ISCO is accomplished by injecting or otherwise introducing oxidizers into the contaminated medium (soil or groundwater) to destroy contaminants. It can be used to remediate a variety of organic compounds, including some that are resistant to natural degradation
Bioremediation
Bioremediation uses microorganisms to remove waste products from a contaminated area. Since 1991 bioremediation has been a suggested tactic to remove impurities such as alkanes, perchlorates, and metals. Bioremediation has seen success because perchlorates are highly soluble, making them difficult to remove.
Hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
() is a common disinfectant that can purify water. It is typically produced at chemical plants and transported to the contaminated water. An alternative approach employs a gold-palladium catalyst to synthesize from ambient hydrogen and oxygen atoms at the use site. The latter was reported to be faster and 107 times more potent at killing ''Escherichia coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escher ...
'' than commercial , and over 108 times more effective than chlorine The catalytic reaction also produces reactive oxygen species (ROS) that bind and degrade other compounds.
Safety and controversies
In April 2007, the water supply of Spencer, Massachusetts
Spencer is a town in Worcester County, Massachusetts, United States. The population was 11,992 at the 2020 census.
For geographic and demographic information on the census-designated place Spencer, please see the article Spencer (CDP), Massach ...
in the United States of America, became contaminated with excess sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
(lye) when its treatment equipment malfunctioned.
Many municipalities have moved from free chlorine to chloramine as a disinfection agent. However, chloramine appears to be a corrosive agent in some water systems. Chloramine can dissolve the "protective" film inside older service lines, leading to the leaching of lead into residential spigots. This can result in harmful exposure, including elevated blood lead levels
Blood lead level (BLL), is a measure of the amount of lead in the blood. Lead is a toxic heavy metal and can cause neurological damage, especially among children, at any detectable level. High lead levels cause decreased vitamin D and haemoglobi ...
. Lead is a known neurotoxin
Neurotoxins are toxins that are destructive to nerve tissue (causing neurotoxicity). Neurotoxins are an extensive class of exogenous chemical neurological insultsSpencer 2000 that can adversely affect function in both developing and mature ner ...
.
Demineralized water
Distillation removes all minerals from water, and the membrane methods of reverse osmosis and nanofiltration remove most to all minerals. This results in demineralised water which is not considered ideal drinking water
Drinking water is water that is used in drink or food preparation; potable water is water that is safe to be used as drinking water. The amount of drinking water required to maintain good health varies, and depends on physical activity level, a ...
. The World Health Organization has investigated the health effects of demineralised water since 1980. Experiments in humans found that demineralised water increased diuresis
Diuresis () is increased urination (polyuria) or, in the related word senses more often intended, the physiological process that produces such an increase or the administration of medications to encourage that process. It involves extra urine pro ...
and the elimination of electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dis ...
s, with decreased blood serum
Serum () is the fluid and solute component of blood which does not play a role in clotting. It may be defined as blood plasma without the clotting factors, or as blood with all cells and clotting factors removed. Serum includes all proteins not u ...
potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosphe ...
concentration. Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
, calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to ...
, and other minerals in water can help to protect against nutritional deficiency. Demineralized water may also increase the risk from toxic metals because it more readily leaches materials from piping like lead and cadmium, which is prevented by dissolved minerals such as calcium and magnesium. Low-mineral water has been implicated in specific cases of lead poisoning in infants, when lead from pipes leached at especially high rates into the water. Recommendations for magnesium have been put at a minimum of 10 mg/L with 20–30 mg/L optimum; for calcium a 20 mg/L minimum and a 40–80 mg/L optimum, and a total water hardness
Hard water is water that has high mineral content (in contrast with "soft water"). Hard water is formed when water percolates through deposits of limestone, chalk or gypsum, which are largely made up of calcium and magnesium carbonates, bicarbo ...
(adding magnesium and calcium) of 2 to 4 mmol
The mole, symbol mol, is the unit of amount of substance in the International System of Units (SI). The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample. The mole is define ...
/L. At water hardness above 5 mmol/L, higher incidence of gallstones, kidney stones, urinary stones, arthrosis, and arthropathies have been observed.[Kozisek F. (2004)]
Health risks from drinking demineralised water
WHO. Additionally, desalination processes can increase the risk of bacterial contamination.[
Manufacturers of home water distillers claim the opposite—that minerals in water are the cause of many diseases, and that most beneficial minerals come from food, not water.
]
History
 The first experiments into water filtration were made in the 17th century. Sir
The first experiments into water filtration were made in the 17th century. Sir Francis Bacon
Francis Bacon, 1st Viscount St Alban (; 22 January 1561 – 9 April 1626), also known as Lord Verulam, was an English philosopher and statesman who served as Attorney General and Lord Chancellor of England. Bacon led the advancement of both ...
attempted to desalinate
Desalination is a process that takes away mineral components from saline water. More generally, desalination refers to the removal of salts and minerals from a target substance, as in soil desalination, which is an issue for agriculture. Saltwa ...
sea water by passing the flow through a sand filter
Sand filters are used as a step in the water treatment process of water purification.
There are three main types; rapid (gravity) sand filters, upward flow sand filters and slow sand filters. All three methods are used extensively in the water i ...
. Although his experiment did not succeed, it marked the beginning of a new interest in the field. The fathers of microscopy
Microscopy is the technical field of using microscopes to view objects and areas of objects that cannot be seen with the naked eye (objects that are not within the resolution range of the normal eye). There are three well-known branches of micr ...
, Antonie van Leeuwenhoek
Antonie Philips van Leeuwenhoek ( ; ; 24 October 1632 – 26 August 1723) was a Dutch microbiologist and microscopist in the Golden Age of Dutch science and technology. A largely self-taught man in science, he is commonly known as " the ...
and Robert Hooke
Robert Hooke FRS (; 18 July 16353 March 1703) was an English polymath active as a scientist, natural philosopher and architect, who is credited to be one of two scientists to discover microorganisms in 1665 using a compound microscope that ...
, used the newly invented microscope
A microscope () is a laboratory instrument used to examine objects that are too small to be seen by the naked eye. Microscopy is the science of investigating small objects and structures using a microscope. Microscopic means being invisibl ...
to observe for the first time small material particles that lay suspended in the water, laying the groundwork for the future understanding of waterborne pathogens.
Sand filter
 The first documented use of
The first documented use of sand filter
Sand filters are used as a step in the water treatment process of water purification.
There are three main types; rapid (gravity) sand filters, upward flow sand filters and slow sand filters. All three methods are used extensively in the water i ...
s to purify the water supply dates to 1804, when the owner of a bleachery in Paisley, Scotland, John Gibb, installed an experimental filter, selling his unwanted surplus to the public.[History of the Chelsea Waterworks](_blank)
ucla.edu This installation provided filtered water for every resident of the area, and the network design was widely copied throughout the United Kingdom in the ensuing decades.
The practice of water treatment soon became mainstream and common, and the virtues of the system were made starkly apparent after the investigations of the physician John Snow
John Snow (15 March 1813 – 16 June 1858) was an English physician and a leader in the development of anaesthesia and medical hygiene. He is considered one of the founders of modern epidemiology, in part because of his work in tracing the so ...
during the 1854 Broad Street cholera outbreak
Events
January–March
* January 4 – The McDonald Islands are discovered by Captain William McDonald aboard the ''Samarang''.
* January 6 – The fictional detective Sherlock Holmes is perhaps born.
* January 9 – The ...
. Snow was sceptical of the then-dominant miasma theory
The miasma theory (also called the miasmatic theory) is an obsolete medical theory that held that diseases—such as cholera, chlamydia, or the Black Death—were caused by a ''miasma'' (, Ancient Greek for 'pollution'), a noxious form of "bad ...
that stated that diseases were caused by noxious "bad airs". Although the germ theory of disease
The germ theory of disease is the currently accepted scientific theory for many diseases. It states that microorganisms known as pathogens or "germs" can lead to disease. These small organisms, too small to be seen without magnification, invade h ...
had not yet been developed, Snow's observations led him to discount the prevailing theory. His 1855 essay ''On the Mode of Communication of Cholera'' conclusively demonstrated the role of the water supply in spreading the cholera epidemic in Soho
Soho is an area of the City of Westminster, part of the West End of London. Originally a fashionable district for the aristocracy, it has been one of the main entertainment districts in the capital since the 19th century.
The area was develop ...
, with the use of a dot distribution map
A dot distribution map (or a dot density map or simply a dot map) is a type of thematic map that uses a point symbol to visualize the geographic distribution of a large number of related phenomena. Dot maps are a type of unit visualizations that ...
and statistical proof to illustrate the connection between the quality of the water source and cholera cases. His data convinced the local council to disable the water pump, which promptly ended the outbreak.
The Metropolis Water Act introduced the regulation of the water supply
Water supply is the provision of water by public utilities, commercial organisations, community endeavors or by individuals, usually via a system of pumps and pipes. Public water supply systems are crucial to properly functioning societies. Thes ...
companies in London, including minimum standards of water quality for the first time. The Act "made provision for securing the supply to the Metropolis of pure and wholesome water", and required that all water be "effectually filtered" from 31 December 1855. This was followed up with legislation for the mandatory inspection of water quality, including comprehensive chemical analyses, in 1858. This legislation set a worldwide precedent for similar state public health interventions across Europe. The Metropolitan Commission of Sewers was formed at the same time, water filtration was adopted throughout the country, and new water intakes on the Thames
The River Thames ( ), known alternatively in parts as the River Isis, is a river that flows through southern England including London. At , it is the longest river entirely in England and the second-longest in the United Kingdom, after the R ...
were established above Teddington Lock
Teddington Lock is a complex of three lock (water transport), locks and a weir on the River Thames between Ham, London, Ham and Teddington in the London Borough of Richmond upon Thames, England. Historically in Middlesex, it was first buil ...
. Automatic pressure filters, where the water is forced under pressure through the filtration system, were innovated in 1899 in England.
Water chlorination
John Snow
John Snow (15 March 1813 – 16 June 1858) was an English physician and a leader in the development of anaesthesia and medical hygiene. He is considered one of the founders of modern epidemiology, in part because of his work in tracing the so ...
was the first to successfully use chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
to disinfect the water supply in Soho
Soho is an area of the City of Westminster, part of the West End of London. Originally a fashionable district for the aristocracy, it has been one of the main entertainment districts in the capital since the 19th century.
The area was develop ...
that had helped spread the cholera outbreak. William Soper also used chlorinated lime to treat the sewage produced by typhoid
Typhoid fever, also known as typhoid, is a disease caused by '' Salmonella'' serotype Typhi bacteria. Symptoms vary from mild to severe, and usually begin six to 30 days after exposure. Often there is a gradual onset of a high fever over several ...
patients in 1879.
In a paper published in 1894, Moritz Traube
Moritz Traube (12 February 1826 in Ratibor, Province of Silesia, Prussia (now Racibórz, Poland) – 28 June 1894 in Berlin, German Empire) was a German chemist (physiological chemistry) and universal private scholar.
Traube worked on chemical, ...
formally proposed the addition of chloride of lime (calcium hypochlorite
Calcium hypochlorite is an inorganic compound with formula Ca(OCl)2. It is the main active ingredient of commercial products called bleaching powder, chlorine powder, or chlorinated lime, used for water treatment and as a bleaching agent. Thi ...
) to water to render it "germ-free." Two other investigators confirmed Traube's findings and published their papers in 1895. Early attempts at implementing water chlorination at a water treatment plant were made in 1893 in Hamburg
(male), (female) en, Hamburger(s),
Hamburgian(s)
, timezone1 = Central (CET)
, utc_offset1 = +1
, timezone1_DST = Central (CEST)
, utc_offset1_DST = +2
, postal ...
, Germany and in 1897 the city of Maidstone, England was the first to have its entire water supply treated with chlorine.
Permanent water chlorination began in 1905, when a faulty slow sand filter and a contaminated water supply led to a serious typhoid fever epidemic in Lincoln, England
Lincoln () is a cathedral city, a non-metropolitan district, and the county town of Lincolnshire, England. In the 2021 Census, the Lincoln district had a population of 103,813. The 2011 census gave the Lincoln Urban Area, urban area of Lincoln, ...
. Dr. Alexander Cruickshank Houston used chlorination of the water to stem the epidemic. His installation fed a concentrated solution of chloride of lime to the water being treated. The chlorination of the water supply helped stop the epidemic and as a precaution, the chlorination was continued until 1911 when a new water supply was instituted.
 The first continuous use of chlorine in the United States for disinfection took place in 1908 at Boonton Reservoir (on the
The first continuous use of chlorine in the United States for disinfection took place in 1908 at Boonton Reservoir (on the Rockaway River
The Rockaway River is a tributary of the Passaic River, approximately 35 mi (56 km) long, in northern New Jersey in the United States. The upper course of the river flows through a wooded mountainous valley, whereas the lower course flo ...
), which served as the supply for Jersey City, New Jersey
Jersey City is the second-most populous city in the U.S. state of New Jersey, after Newark.[calcium hypochlorite
Calcium hypochlorite is an inorganic compound with formula Ca(OCl)2. It is the main active ingredient of commercial products called bleaching powder, chlorine powder, or chlorinated lime, used for water treatment and as a bleaching agent. Thi ...]
) at doses of 0.2 to 0.35 ppm. The treatment process was conceived by Dr. John L. Leal and the chlorination plant was designed by George Warren Fuller. Over the next few years, chlorine disinfection using chloride of lime were rapidly installed in drinking water systems around the world.[Hazen, Allen. (1916). ''Clean Water and How to Get It.'' New York:Wiley. p. 102.]
The technique of purification of drinking water by use of compressed liquefied chlorine gas was developed by a British officer in the Indian Medical Service, Vincent B. Nesfield, in 1903. According to his own account:
U.S. Army Major Carl Rogers Darnall
Brigadier General Carl Rogers Darnall (December 25, 1867 in Weston, Texas – January 18, 1941 in Washington, D.C.) was a United States Army chemist and surgeon credited with originating the technique of liquid chlorination of drinking wate ...
, Professor of Chemistry at the Army Medical School
Founded by U.S. Army Brigadier General George Miller Sternberg, MD in 1893, the Army Medical School (AMS) was by some reckonings the world's first school of public health and preventive medicine. (The other institution vying for this distinction ...
, gave the first practical demonstration of this in 1910. Shortly thereafter, Major William J. L. Lyster of the Army Medical Department used a solution of calcium hypochlorite
Calcium hypochlorite is an inorganic compound with formula Ca(OCl)2. It is the main active ingredient of commercial products called bleaching powder, chlorine powder, or chlorinated lime, used for water treatment and as a bleaching agent. Thi ...
in a linen bag to treat water. For many decades, Lyster's method remained the standard for U.S. ground forces in the field and in camps, implemented in the form of the familiar Lyster Bag (also spelled Lister Bag). The bag was made of canvas and could hold 36 gallons of water. It was porous and held up by ropes, purifying water with the help of calcium hypochlorite solution. Each bag had a faucet attached, which was used to flush water for testing, as well as dispensing for use. This became the basis for present day systems of municipal water ''purification''.
See also
* History of water supply and sanitation
* List of water supply and sanitation by country
* Microfiltration
* Organisms involved in water purification
*Portable water purification
Portable water purification devices are self-contained, easily transported units used to purify water from untreated sources (such as rivers, lakes, and wells) for drinking purposes. Their main function is to eliminate pathogens, and often als ...
*Water softening
Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions. Soft water also extend ...
*Water conservation
Water conservation includes all the policies, strategies and activities to sustainably manage the natural resource of fresh water, to protect the hydrosphere, and to meet the current and future human demand (thus avoiding water scarcity). Populati ...
*Water recycling
Water reclamation (also called wastewater reuse, water reuse or water recycling) is the process of converting municipal wastewater (sewage) or industrial wastewater into water that can be reused for a variety of purposes. Types of reuse include: ...
*Water treatment
Water treatment is any process that improves the Water quality, quality of water to make it appropriate for a specific end-use. The end use may be drinking water, drinking, industrial water supply, irrigation, river flow maintenance, water recrea ...
References
Further reading
*
*
* EPA
"Ground Water and Drinking Water"
Overview and detailed information on US regulatory program and related drinking water topics
External links
American Water Works Association
"Water On Tap: What You Need To Know."
– Consumer Guide to Drinking Water in the US (EPA)
* ttp://ecfr.gpoaccess.gov/cgi/t/text/text-idx?c=ecfr&sid=39f1366979712eca0ac4e22f5f52b290&tpl=/ecfrbrowse/Title40/40cfr141_main_02.tpl Code of Federal Regulations, Title 40, Part 141 – U.S. National Primary Drinking Water Regulations
{{DEFAULTSORT:Water Purification
Water technology
Water pollution
Water treatment
 Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from
Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from 
 One of the first steps in most conventional water purification processes is the addition of chemicals to assist in the removal of particles suspended in water. Particles can be inorganic such as
One of the first steps in most conventional water purification processes is the addition of chemicals to assist in the removal of particles suspended in water. Particles can be inorganic such as  The most common type of filter is a
The most common type of filter is a 
 The first experiments into water filtration were made in the 17th century. Sir
The first experiments into water filtration were made in the 17th century. Sir  The first documented use of
The first documented use of  The first continuous use of chlorine in the United States for disinfection took place in 1908 at Boonton Reservoir (on the
The first continuous use of chlorine in the United States for disinfection took place in 1908 at Boonton Reservoir (on the