Einsteinium on:
[Wikipedia]
[Google]
[Amazon]
Einsteinium is a
 Einsteinium was first identified in December 1952 by
Einsteinium was first identified in December 1952 by ^_U -> ce-2\ \beta^-] ^_Pu
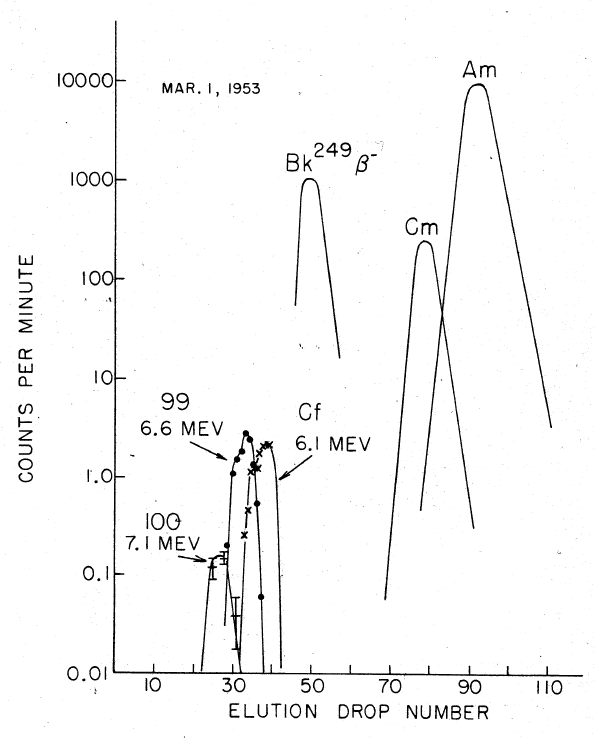
At the time, the multiple neutron absorption was thought to be an extremely rare process, but the identification of indicated that still more neutrons could have been captured by the uranium nuclei, thereby producing new elements heavier than  Ghiorso and co-workers analyzed filter papers which had been flown through the explosion cloud on airplanes (the same sampling technique that had been used to discover ). #Seaborg, Seaborg, p. 39 Larger amounts of radioactive material were later isolated from coral debris of the atoll, which were delivered to the U.S. The separation of suspected new elements was carried out in the presence of a
Ghiorso and co-workers analyzed filter papers which had been flown through the explosion cloud on airplanes (the same sampling technique that had been used to discover ). #Seaborg, Seaborg, p. 39 Larger amounts of radioactive material were later isolated from coral debris of the atoll, which were delivered to the U.S. The separation of suspected new elements was carried out in the presence of a
Nature's building blocks: an A-Z guide to the elements
, Oxford University Press, 2003, pp. 133–135 Nevertheless, element 99 (einsteinium), namely its 253Es isotope, could be detected via its characteristic high-energy
^_U -> ce6 \beta^-] ^_Cf -> beta^-^_Es
Some 238U atoms, however, could absorb two additional neutrons (for a total of 17), resulting in 255Es, as well as in the 255Fm isotope of another new element,
However, the rapid capture of so many neutrons would provide needed direct experimental confirmation of the so-called^_Cf -> ce^_Cf -> beta^-17.81 \ce] ^_Es -> ce^_Es -> beta^-^_Fm
These results were published in several articles in 1954 with the disclaimer that these were not the first studies that had been carried out on the elements. The Berkeley team also reported some results on the chemical properties of einsteinium and fermium.Seaborg, G. T.; Thompson, S.G.; Harvey, B.G. and Choppin, G.R. (July 23, 1954
"Chemical Properties of Elements 99 and 100"
, Radiation Laboratory, University of California, Berkeley, UCRL-2591 The ''Ivy Mike'' results were declassified and published in 1955. In their discovery of the elements 99 and 100, the American teams had competed with a group at the Nobel Institute for Physics,
In their discovery of the elements 99 and 100, the American teams had competed with a group at the Nobel Institute for Physics,
Modern alchemy: selected papers of Glenn T. Seaborg
'', World Scientific, p. 6, .
 Einsteinium is a synthetic, silver, radioactive metal. In the
Einsteinium is a synthetic, silver, radioactive metal. In the
Further, owing to the small size of the available samples, the melting point of einsteinium was often deduced by observing the sample being heated inside an electron microscope. #Seaborg, Seaborg, p. 61 Thus, the surface effects in small samples could reduce the melting point value. The metal is trivalent and has a noticeably high volatility. In order to reduce the self-radiation damage, most measurements of solid einsteinium and its compounds are performed right after thermal annealing. #Seaborg, Seaborg, p. 52 Also, some compounds are studied under the atmosphere of the reductant gas, for example H2O+
^_Es ->
Thus, most einsteinium samples are contaminated, and their intrinsic properties are often deduced by extrapolating back experimental data accumulated over time. Other experimental techniques to circumvent the contamination problem include selective optical excitation of einsteinium ions by a tunable laser, such as in studying its luminescence properties. #Seaborg, Seaborg, p. 76
Magnetic properties have been studied for einsteinium metal, its oxide and fluoride. All three materials showed Curie–Weiss law, Curie–Weiss
"Evaluation of nuclear criticality safety data and limits for actinides in transport"
, p. 16.
 Einsteinium is produced in minute quantities by bombarding lighter actinides with neutrons in dedicated high-flux
Einsteinium is produced in minute quantities by bombarding lighter actinides with neutrons in dedicated high-flux
^_Bk -> \alpha^_Es
Einsteinium-253 was produced by irradiating a 0.1–0.2 milligram 252Cf target with a ^_Cf -> ce^_Cf -> beta^-17.81 \ce] ^_Es
In 2020, scientists at the Oak Ridge National Laboratory were able to create 233 nanograms of 254Es, a new world record. This allowed some chemical properties of the element to be studied for the first time.
 The analysis of the debris at the 10- TNT equivalent, megaton ''Ivy Mike'' nuclear test was a part of long-term project. One of the goals of which was studying the efficiency of production of transuranium elements in high-power nuclear explosions. The motivation for these experiments was that synthesis of such elements from uranium requires multiple neutron capture. The probability of such events increases with the
The analysis of the debris at the 10- TNT equivalent, megaton ''Ivy Mike'' nuclear test was a part of long-term project. One of the goals of which was studying the efficiency of production of transuranium elements in high-power nuclear explosions. The motivation for these experiments was that synthesis of such elements from uranium requires multiple neutron capture. The probability of such events increases with the
 Separation procedure of einsteinium depends on the synthesis method. In the case of light-ion bombardment inside a cyclotron, the heavy ion target is attached to a thin foil, and the generated einsteinium is simply washed off the foil after the irradiation. However, the produced amounts in such experiments are relatively low. Haire, p. 1583 The yields are much higher for reactor irradiation, but there, the product is a mixture of various actinide isotopes, as well as lanthanides produced in the nuclear fission decays. In this case, isolation of einsteinium is a tedious procedure which involves several repeating steps of cation exchange, at elevated temperature and pressure, and chromatography. Separation from berkelium is important, because the most common einsteinium isotope produced in nuclear reactors, 253Es, decays with a half-life of only 20 days to 249Bk, which is fast on the timescale of most experiments. Such separation relies on the fact that berkelium easily oxidizes to the solid +4 state and precipitates, whereas other actinides, including einsteinium, remain in their +3 state in solutions. Haire, pp. 1584–1585
Separation of trivalent actinides from lanthanide fission products can be done by a cation-exchange resin column using a 90% water/10% ethanol solution saturated with
Separation procedure of einsteinium depends on the synthesis method. In the case of light-ion bombardment inside a cyclotron, the heavy ion target is attached to a thin foil, and the generated einsteinium is simply washed off the foil after the irradiation. However, the produced amounts in such experiments are relatively low. Haire, p. 1583 The yields are much higher for reactor irradiation, but there, the product is a mixture of various actinide isotopes, as well as lanthanides produced in the nuclear fission decays. In this case, isolation of einsteinium is a tedious procedure which involves several repeating steps of cation exchange, at elevated temperature and pressure, and chromatography. Separation from berkelium is important, because the most common einsteinium isotope produced in nuclear reactors, 253Es, decays with a half-life of only 20 days to 249Bk, which is fast on the timescale of most experiments. Such separation relies on the fact that berkelium easily oxidizes to the solid +4 state and precipitates, whereas other actinides, including einsteinium, remain in their +3 state in solutions. Haire, pp. 1584–1585
Separation of trivalent actinides from lanthanide fission products can be done by a cation-exchange resin column using a 90% water/10% ethanol solution saturated with
 Einsteinium
Einsteinium
:2 EsX3 + H2 → 2 EsX2 + 2 HX, X = F, Cl, Br, I Einsteinium(II) chloride (EsCl2), einsteinium(II) bromide (EsBr2), and einsteinium(II) iodide (EsI2) have been produced and characterized by optical absorption, with no structural information available yet. Known oxyhalides of einsteinium include EsOCl, EsOBr and EsOI. These salts are synthesized by treating a trihalide with a vapor mixture of water and the corresponding hydrogen halide: for example, EsCl3 + H2O/HCl to obtain EsOCl. #Seaborg, Seaborg, p. 60
+ -> -> no\ atoms
254Es was used as the calibration marker in the chemical analysis spectrometer (" alpha-scattering surface analyzer") of the
Einsteinium
at ''
Age-related factors in radionuclide metabolism and dosimetry: Proceedings
– contains several health related studies of einsteinium {{Authority control Chemical elements Chemical elements with face-centered cubic structure Actinides Synthetic elements Albert Einstein
synthetic element
A synthetic element is one of 24 known chemical elements that do not occur naturally on Earth: they have been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; ...
with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Es and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
99. Einsteinium is a member of the actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The inform ...
series and it is the seventh transuranium element
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. ...
. It was named in honor of Albert Einstein
Albert Einstein ( ; ; 14 March 1879 – 18 April 1955) was a German-born theoretical physicist, widely acknowledged to be one of the greatest and most influential physicists of all time. Einstein is best known for developing the theory ...
.
Einsteinium was discovered as a component of the debris of the first hydrogen bomb explosion in 1952. Its most common isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) ...
, einsteinium-253 (half-life 20.47 days), is produced artificially from decay of californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
-253 in a few dedicated high-power nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nu ...
s with a total yield on the order of one milligram per year. The reactor synthesis is followed by a complex process of separating einsteinium-253 from other actinides and products of their decay. Other isotopes are synthesized in various laboratories, but in much smaller amounts, by bombarding heavy actinide elements with light ions. Owing to the small amounts of produced einsteinium and the short half-life of its most easily produced isotope, there are currently almost no practical applications for it outside basic scientific research. In particular, einsteinium was used to synthesize, for the first time, 17 atoms of the new element mendelevium
Mendelevium is a synthetic element with the symbol Md ( formerly Mv) and atomic number 101. A metallic radioactive transuranium element in the actinide series, it is the first element by atomic number that currently cannot be produced in macroscopi ...
in 1955.
Einsteinium is a soft, silvery, paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, d ...
metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
. Its chemistry is typical of the late actinides, with a preponderance of the +3 oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
; the +2 oxidation state is also accessible, especially in solids. The high radioactivity of einsteinium-253 produces a visible glow and rapidly damages its crystalline metal lattice, with released heat of about 1000 watt
The watt (symbol: W) is the unit of power or radiant flux in the International System of Units (SI), equal to 1 joule per second or 1 kg⋅m2⋅s−3. It is used to quantify the rate of energy transfer. The watt is named after James Wa ...
s per gram. Difficulty in studying its properties is due to einsteinium-253's decay to berkelium
Berkelium is a transuranic radioactive chemical element with the symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Ber ...
-249 and then californium-249 at a rate of about 3% per day. The isotope of einsteinium with the longest half-life, einsteinium-252 (half-life 471.7 days) would be more suitable for investigation of physical properties, but it has proven far more difficult to produce and is available only in minute quantities, and not in bulk. Einsteinium is the element with the highest atomic number which has been observed in macroscopic quantities in its pure form and this was the common short-lived isotope einsteinium-253.
Like all synthetic transuranium elements, isotopes of einsteinium are very radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
and are considered highly dangerous to health on ingestion.
History
 Einsteinium was first identified in December 1952 by
Einsteinium was first identified in December 1952 by Albert Ghiorso
Albert Ghiorso (July 15, 1915 – December 26, 2010) was an American nuclear scientist and co-discoverer of a record 12 chemical elements on the periodic table. His research career spanned six decades, from the early 1940s to the late 1990s.
Biog ...
and co-workers at the University of California, Berkeley
The University of California, Berkeley (UC Berkeley, Berkeley, Cal, or California) is a public land-grant research university in Berkeley, California. Established in 1868 as the University of California, it is the state's first land-grant u ...
in collaboration with the Argonne and Los Alamos National Laboratories, in the fallout from the ''Ivy Mike
Ivy Mike was the codename given to the first full-scale test of a thermonuclear device, in which part of the explosive yield comes from nuclear fusion.
Ivy Mike was detonated on November 1, 1952, by the United States on the island of Elugelab in ...
'' nuclear test. The test was carried out on November 1, 1952, at Enewetak Atoll
Enewetak Atoll (; also spelled Eniwetok Atoll or sometimes Eniewetok; mh, Ānewetak, , or , ; known to the Japanese as Brown Atoll or Brown Island; ja, ブラウン環礁) is a large coral atoll of 40 islands in the Pacific Ocean and with it ...
in the Pacific Ocean
The Pacific Ocean is the largest and deepest of Earth's five oceanic divisions. It extends from the Arctic Ocean in the north to the Southern Ocean (or, depending on definition, to Antarctica) in the south, and is bounded by the continen ...
and was the first successful test of a thermonuclear weapon
A thermonuclear weapon, fusion weapon or hydrogen bomb (H bomb) is a second-generation nuclear weapon design. Its greater sophistication affords it vastly greater destructive power than first-generation nuclear bombs, a more compact size, a lowe ...
. Initial examination of the debris from the explosion had shown the production of a new isotope of plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibi ...
, , which could only have formed by the absorption of six neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
s by a uranium-238
Uranium-238 (238U or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it ...
nucleus followed by two beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
s.
:californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
.
 Ghiorso and co-workers analyzed filter papers which had been flown through the explosion cloud on airplanes (the same sampling technique that had been used to discover ). #Seaborg, Seaborg, p. 39 Larger amounts of radioactive material were later isolated from coral debris of the atoll, which were delivered to the U.S. The separation of suspected new elements was carried out in the presence of a
Ghiorso and co-workers analyzed filter papers which had been flown through the explosion cloud on airplanes (the same sampling technique that had been used to discover ). #Seaborg, Seaborg, p. 39 Larger amounts of radioactive material were later isolated from coral debris of the atoll, which were delivered to the U.S. The separation of suspected new elements was carried out in the presence of a citric acid
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in t ...
/ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
buffer solution
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is ...
in a weakly acidic medium ( pH ≈ 3.5), using ion exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
at elevated temperatures; fewer than 200 atoms of einsteinium were recovered in the end.John EmsleNature's building blocks: an A-Z guide to the elements
, Oxford University Press, 2003, pp. 133–135 Nevertheless, element 99 (einsteinium), namely its 253Es isotope, could be detected via its characteristic high-energy
alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an atom ...
at 6.6 MeV. It was produced by the capture of 15 neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
s by uranium-238
Uranium-238 (238U or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it ...
nuclei followed by seven beta-decays, and had a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ato ...
of 20.5 days. Such multiple neutron absorption was made possible by the high neutron flux density during the detonation, so that newly generated heavy isotopes had plenty of available neutrons to absorb before they could disintegrate into lighter elements. Neutron capture initially raised the mass number
The mass number (symbol ''A'', from the German word ''Atomgewicht'' tomic weight, also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approxima ...
without changing the atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
of the nuclide, and the concomitant beta-decays resulted in a gradual increase in the atomic number:
:fermium
Fermium is a synthetic element with the symbol Fm and atomic number 100. It is an actinide and the heaviest element that can be formed by neutron bombardment of lighter elements, and hence the last element that can be prepared in macroscopic qua ...
. The discovery of the new elements and the associated new data on multiple neutron capture were initially kept secret on the orders of the U.S. military until 1955 due to Cold War
The Cold War is a term commonly used to refer to a period of geopolitical tension between the United States and the Soviet Union and their respective allies, the Western Bloc and the Eastern Bloc. The term '' cold war'' is used because the ...
tensions and competition with Soviet Union in nuclear technologies.Google BooksHowever, the rapid capture of so many neutrons would provide needed direct experimental confirmation of the so-called
r-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for the creation of approximately half of the atomic nuclei heavier than iron, the "heavy elements", ...
multiple neutron absorption needed to explain the cosmic nucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in ...
(production) of certain heavy chemical elements (heavier than nickel) in supernova
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or when ...
explosions, before beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
. Such a process is needed to explain the existence of many stable elements in the universe.
Meanwhile, isotopes of element 99 (as well as of new element 100, fermium
Fermium is a synthetic element with the symbol Fm and atomic number 100. It is an actinide and the heaviest element that can be formed by neutron bombardment of lighter elements, and hence the last element that can be prepared in macroscopic qua ...
) were produced in the Berkeley and Argonee laboratories, in a nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two atomic nucleus, nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a t ...
between nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
-14 and uranium-238, and later by intense neutron irradiation of plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibi ...
or californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
:
:"Chemical Properties of Elements 99 and 100"
, Radiation Laboratory, University of California, Berkeley, UCRL-2591 The ''Ivy Mike'' results were declassified and published in 1955.
 In their discovery of the elements 99 and 100, the American teams had competed with a group at the Nobel Institute for Physics,
In their discovery of the elements 99 and 100, the American teams had competed with a group at the Nobel Institute for Physics, Stockholm
Stockholm () is the Capital city, capital and List of urban areas in Sweden by population, largest city of Sweden as well as the List of urban areas in the Nordic countries, largest urban area in Scandinavia. Approximately 980,000 people liv ...
, Sweden
Sweden, formally the Kingdom of Sweden,The United Nations Group of Experts on Geographical Names states that the country's formal name is the Kingdom of SwedenUNGEGN World Geographical Names, Sweden./ref> is a Nordic country located on ...
. In late 1953 – early 1954, the Swedish group succeeded in the synthesis of light isotopes of element 100, in particular 250Fm, by bombarding uranium with oxygen nuclei. These results were also published in 1954. Nevertheless, the priority of the Berkeley team was generally recognized, as its publications preceded the Swedish article, and they were based on the previously undisclosed results of the 1952 thermonuclear explosion; thus the Berkeley team was given the privilege to name the new elements. As the effort which had led to the design of ''Ivy Mike'' was codenamed Project PANDA, element 99 had been jokingly nicknamed "Pandemonium" but the official names suggested by the Berkeley group derived from two prominent scientists, Albert Einstein
Albert Einstein ( ; ; 14 March 1879 – 18 April 1955) was a German-born theoretical physicist, widely acknowledged to be one of the greatest and most influential physicists of all time. Einstein is best known for developing the theory ...
and Enrico Fermi
Enrico Fermi (; 29 September 1901 – 28 November 1954) was an Italian (later naturalized American) physicist and the creator of the world's first nuclear reactor, the Chicago Pile-1. He has been called the "architect of the nuclear age" and ...
: "We suggest for the name for the element with the atomic number 99, einsteinium (symbol E) after Albert Einstein and for the name for the element with atomic number 100, fermium (symbol Fm), after Enrico Fermi." Both Einstein and Fermi died between the time the names were originally proposed and when they were announced. The discovery of these new elements was announced by Albert Ghiorso
Albert Ghiorso (July 15, 1915 – December 26, 2010) was an American nuclear scientist and co-discoverer of a record 12 chemical elements on the periodic table. His research career spanned six decades, from the early 1940s to the late 1990s.
Biog ...
at the first Geneva Atomic Conference held on 8–20 August 1955. The symbol for einsteinium was first given as "E" and later changed to "Es" by IUPAC. Haire, p. 1577Seaborg, G.T. (1994) Modern alchemy: selected papers of Glenn T. Seaborg
'', World Scientific, p. 6, .
Characteristics
Physical
periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
, it is located to the right of the actinide californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
, to the left of the actinide fermium
Fermium is a synthetic element with the symbol Fm and atomic number 100. It is an actinide and the heaviest element that can be formed by neutron bombardment of lighter elements, and hence the last element that can be prepared in macroscopic qua ...
and below the lanthanide holmium
Holmium is a chemical element with the symbol Ho and atomic number 67. It is a rare-earth element and the eleventh member of the lanthanide series. It is a relatively soft, silvery, fairly corrosion-resistant and malleable metal. Like a lot of othe ...
with which it shares many similarities in physical and chemical properties. Its density of 8.84 g/cm3 is lower than that of californium (15.1 g/cm3) and is nearly the same as that of holmium (8.79 g/cm3), despite atomic einsteinium being much heavier than holmium. The melting point of einsteinium (860 °C) is also relatively low – below californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
(900 °C), fermium
Fermium is a synthetic element with the symbol Fm and atomic number 100. It is an actinide and the heaviest element that can be formed by neutron bombardment of lighter elements, and hence the last element that can be prepared in macroscopic qua ...
(1527 °C) and holmium (1461 °C).Hammond C. R. "The elements" in Haire, R. G. (1990) "Properties of the Transplutonium Metals (Am-Fm)", in: Metals Handbook, Vol. 2, 10th edition, (ASM International, Materials Park, Ohio), pp. 1198–1201. Einsteinium is a soft metal, with the bulk modulus
The bulk modulus (K or B) of a substance is a measure of how resistant to compression the substance is. It is defined as the ratio of the infinitesimal pressure increase to the resulting ''relative'' decrease of the volume.
Other moduli describe ...
of only 15 GPa, which value is one of the lowest among non-alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s. Haire, p. 1591
Contrary to the lighter actinides californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
, berkelium
Berkelium is a transuranic radioactive chemical element with the symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Ber ...
, curium
Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first inte ...
and americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was na ...
, which crystallize in a double hexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A '' regular hexagon'' has ...
structure at ambient conditions, einsteinium is believed to have a face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
(''fcc'') symmetry with the space group ''Fm'm'' and the lattice constant ''a'' = 575 pm. However, there is a report of room-temperature hexagonal einsteinium metal with ''a'' = 398 pm and ''c'' = 650 pm, which converted to the ''fcc'' phase upon heating to 300 °C.
The self-damage induced by the radioactivity of einsteinium is so strong that it rapidly destroys the crystal lattice, and the energy release during this process, 1000 watts per gram of 253Es, induces a visible glow. Haire, p. 1579 These processes may contribute to the relatively low density and melting point of einsteinium.draft manuscriptFurther, owing to the small size of the available samples, the melting point of einsteinium was often deduced by observing the sample being heated inside an electron microscope. #Seaborg, Seaborg, p. 61 Thus, the surface effects in small samples could reduce the melting point value. The metal is trivalent and has a noticeably high volatility. In order to reduce the self-radiation damage, most measurements of solid einsteinium and its compounds are performed right after thermal annealing. #Seaborg, Seaborg, p. 52 Also, some compounds are studied under the atmosphere of the reductant gas, for example H2O+
HCl HCL may refer to:
Science and medicine
* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia
* Harvard Cyclotron Laboratory, from 1961 to 2002, a proton accelerator used for research and development
* Hollow-cathode lamp, a spe ...
for EsOCl so that the sample is partly regrown during its decomposition.
Apart from the self-destruction of solid einsteinium and its compounds, other intrinsic difficulties in studying this element include scarcity – the most common 253Es isotope is available only once or twice a year in sub-milligram amounts – and self-contamination due to rapid conversion of einsteinium to berkelium and then to californium at a rate of about 3.3% per day: #Seaborg, Seaborg, p. 55
:alpha
Alpha (uppercase , lowercase ; grc, ἄλφα, ''álpha'', or ell, άλφα, álfa) is the first letter of the Greek alphabet. In the system of Greek numerals, it has a value of one. Alpha is derived from the Phoenician letter aleph , whic ...
20 \ce] ^_Bk -> beta^-314 \ce] ^_Cf
paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, d ...
behavior from liquid helium
Liquid helium is a physical state of helium at very low temperatures at standard atmospheric pressures. Liquid helium may show superfluidity.
At standard pressure, the chemical element helium exists in a liquid form only at the extremely low temp ...
to room temperature. The effective magnetic moments were deduced as for Es2O3 and for the EsF3, which are the highest values among actinides, and the corresponding Curie temperature
In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cur ...
s are 53 and 37 K.
Chemical
Like all actinides, einsteinium is rather reactive. Its trivalentoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
is most stable in solids and aqueous solution where it induces a pale pink color. Holleman, p. 1956 The existence of divalent einsteinium is firmly established, especially in the solid phase; such +2 state is not observed in many other actinides, including protactinium
Protactinium (formerly protoactinium) is a chemical element with the symbol Pa and atomic number 91. It is a dense, silvery-gray actinide metal which readily reacts with oxygen, water vapor and inorganic acids. It forms various chemical compounds ...
, uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
, neptunium
Neptunium is a chemical element with the Symbol (chemistry), symbol Np and atomic number 93. A radioactivity, radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after ...
, plutonium, curium and berkelium. Einsteinium(II) compounds can be obtained, for example, by reducing einsteinium(III) with samarium(II) chloride
Samarium(II) chloride ( Sm Cl2) is a chemical compound, used as a radical generating agent in the ketone-mediated intraannulation reaction.
Preparation
Reduction of samarium(III) chloride with samarium metal in a vacuum at a temperature of 800&nb ...
. #Seaborg, Seaborg, p. 53 The oxidation state +4 was postulated from vapor studies and is as yet uncertain. Haire, p. 1578
Isotopes
Nineteen isotopes and threenuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state, higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited ...
s are known for einsteinium, with mass number
The mass number (symbol ''A'', from the German word ''Atomgewicht'' tomic weight, also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approxima ...
s ranging from 240 to 257. All are radioactive and the most stable nuclide, 252Es, has a half-life of 471.7 days. The next most stable isotopes are 254Es (half-life 275.7 days), 255Es (39.8 days), and 253Es (20.47 days). All of the remaining isotopes have half-lives shorter than 40 hours, most shorter than 30 minutes. Of the three nuclear isomers, the most stable is 254mEs with a half-life of 39.3 hours.
Nuclear fission
Einsteinium has a high rate ofnuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
that results in a low critical mass
In nuclear engineering, a critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The critical mass of a fissionable material depends upon its nuclear properties (specifically, its nuclear fissi ...
for a sustained nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nu ...
. This mass is 9.89 kilograms for a bare sphere of 254Es isotope, and can be lowered to 2.9 kilograms by adding a 30-centimeter-thick steel neutron reflector
A neutron reflector is any material that reflects neutrons. This refers to elastic scattering rather than to a specular reflection. The material may be graphite, beryllium, steel, tungsten carbide, gold, or other materials. A neutron reflector ...
, or even to 2.26 kilograms with a 20-cm-thick reflector made of water. However, even this small critical mass greatly exceeds the total amount of einsteinium isolated thus far, especially of the rare 254Es isotope.Institut de Radioprotection et de Sûreté Nucléaire"Evaluation of nuclear criticality safety data and limits for actinides in transport"
, p. 16.
Natural occurrence
Because of the short half-life of all isotopes of einsteinium, any primordial einsteinium—that is, einsteinium that could possibly have been present on the Earth during its formation—has long since decayed. Synthesis of einsteinium from naturally-occurring actinides uranium and thorium in the Earth's crust requires multiple neutron capture, which is an extremely unlikely event. Therefore, all terrestrial einsteinium is produced in scientific laboratories, high-power nuclear reactors, or innuclear weapons tests
Nuclear weapons tests are experiments carried out to determine nuclear weapons' effectiveness, yield, and explosive capability. Testing nuclear weapons offers practical information about how the weapons function, how detonations are affected b ...
, and is present only within a few years from the time of the synthesis.
The transuranic elements from americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was na ...
to fermium
Fermium is a synthetic element with the symbol Fm and atomic number 100. It is an actinide and the heaviest element that can be formed by neutron bombardment of lighter elements, and hence the last element that can be prepared in macroscopic qua ...
, including einsteinium, occurred naturally in the natural nuclear fission reactor
A natural nuclear fission reactor is a uranium deposit where self-sustaining nuclear chain reactions occur. The conditions under which a natural nuclear reactor could exist had been predicted in 1956 by Japanese American chemist Paul Kuroda. Th ...
at Oklo
Oklo is a region near the town of Franceville, in the Haut-Ogooué province of the Central African country of Gabon. Several natural nuclear fission reactors were discovered in the uranium mines in the region in 1972.
History
Gabon was a French ...
, but no longer do so.
Einsteinium was theoretically observed in the spectrum of Przybylski's Star. However, the lead author of the studies finding einsteinium and other short-lived actinides in Przybylski's Star, Vera F. Gopka, directly admits that "the position of lines of the radioactive elements under search were simply visualized in synthetic spectrum as vertical markers because there are no any atomic data for these lines except for their wavelengths (Sansonetti et al. 2004), enabling one to calculate their profiles with more or less real intensities." The signature spectra of einsteinium's isotopes have since been comprehensively analyzed experimentally (in 2021), though there is currently no published research confirming whether the theorized einsteinium signatures proposed to be found in the star's spectrum match the lab-determined results.
Synthesis and extraction
 Einsteinium is produced in minute quantities by bombarding lighter actinides with neutrons in dedicated high-flux
Einsteinium is produced in minute quantities by bombarding lighter actinides with neutrons in dedicated high-flux nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nu ...
s. The world's major irradiation sources are the 85-megawatt High Flux Isotope Reactor
The High Flux Isotope Reactor (HFIR) is a nuclear research reactor at Oak Ridge National Laboratory (ORNL) in Oak Ridge, Tennessee, United States. Operating at 85 MW, HFIR is one of the highest flux reactor-based sources of neutrons for condense ...
(HFIR) at the Oak Ridge National Laboratory
Oak Ridge National Laboratory (ORNL) is a U.S. multiprogram science and technology national laboratory sponsored by the U.S. Department of Energy (DOE) and administered, managed, and operated by UT–Battelle as a federally funded research and ...
in Tennessee, U.S., and the SM-2 loop reactor at the Research Institute of Atomic Reactors (NIIAR) in Dimitrovgrad, Russia
Dimitrovgrad (russian: Димитровград; ), formerly Melekess () until 1972, is a city in Ulyanovsk Oblast, Russia. It is the administrative center of Melekessky District, although it is not within the district and is an independent ci ...
, which are both dedicated to the production of transcurium (''Z'' > 96) elements. These facilities have similar power and flux levels, and are expected to have comparable production capacities for transcurium elements, Haire, p. 1582 although the quantities produced at NIIAR are not widely reported. In a "typical processing campaign" at Oak Ridge, tens of grams of curium
Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first inte ...
are irradiated to produce decigram quantities of californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding ...
, milligram quantities of berkelium (249Bk) and einsteinium and picogram quantities of fermium
Fermium is a synthetic element with the symbol Fm and atomic number 100. It is an actinide and the heaviest element that can be formed by neutron bombardment of lighter elements, and hence the last element that can be prepared in macroscopic qua ...
.
The first microscopic sample of 253Es sample weighing about 10 nanogram
To help compare different orders of magnitude, the following lists describe various mass levels between 10−59 kg and 1052 kg. The least massive thing listed here is a graviton, and the most massive thing is the observable universe ...
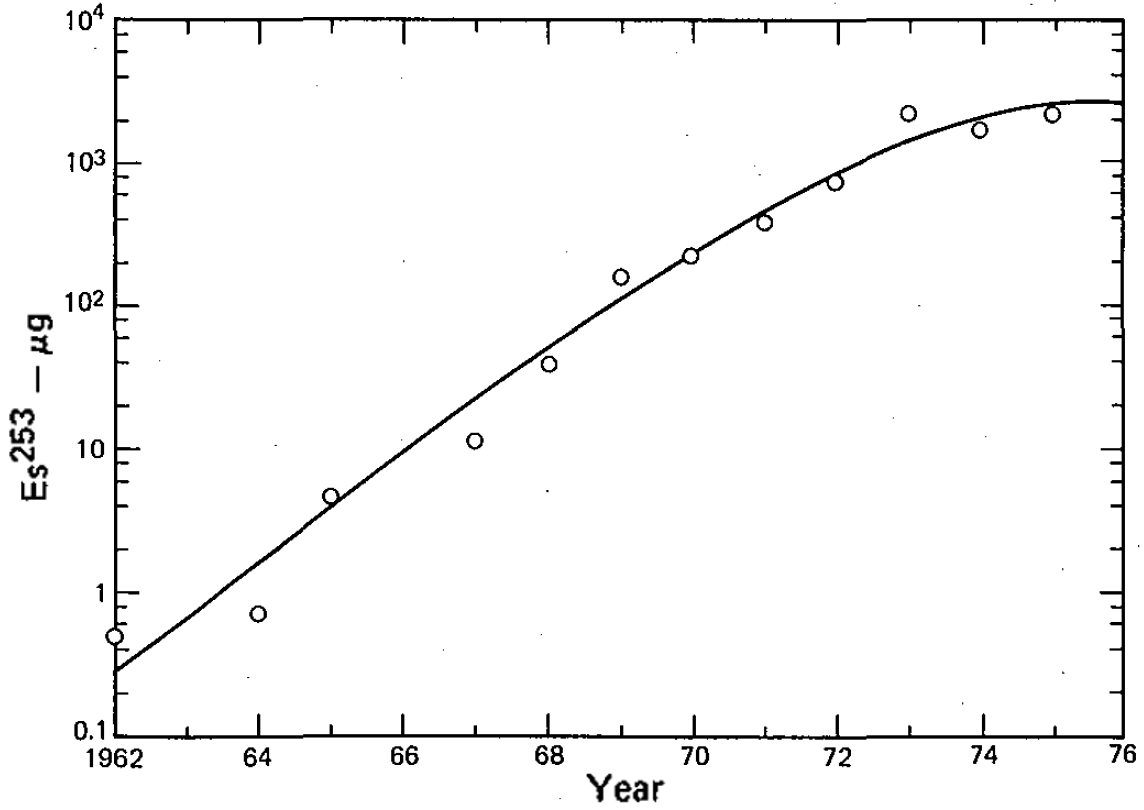
s was prepared in 1961 at HFIR. A special magnetic balance was designed to estimate its weight. Larger batches were produced later starting from several kilograms of plutonium with the einsteinium yields (mostly 253Es) of 0.48 milligrams in 1967–1970, 3.2 milligrams in 1971–1973, followed by steady production of about 3 milligrams per year between 1974 and 1978. #Seaborg, Seaborg, pp. 36–37 These quantities however refer to the integral amount in the target right after irradiation. Subsequent separation procedures reduced the amount of isotopically pure einsteinium roughly tenfold.
Laboratory synthesis
Heavy neutron irradiation of plutonium results in four major isotopes of einsteinium: 253Es (α-emitter with half-life of 20.47 days and with a spontaneous fission half-life of 7×105 years); 254''m''Es (β-emitter with half-life of 39.3 hours), 254Es (α-emitter with half-life of about 276 days) and 255Es (β-emitter with half-life of 39.8 days). An alternative route involves bombardment of uranium-238 with high-intensity nitrogen or oxygen ion beams. Einsteinium-247 (half-life 4.55 minutes) was produced by irradiating americium-241 with carbon or uranium-238 with nitrogen ions.Harry H. Binder: ''Lexikon der chemischen Elemente'', S. Hirzel Verlag, Stuttgart 1999, , pp. 18–23. The latter reaction was first realized in 1967 in Dubna, Russia, and the involved scientists were awarded theLenin Komsomol Prize
Lenin Komsomol Prize () was a Soviet annual award for the best works in science, engineering, literature or art carried out by young authors of age not exceeding 33 years. Komsomol was the abbreviated name of The Communist Union of Youth (Russia ...
.
The isotope 248Es was produced by irradiating 249Cf with deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two Stable isotope ratio, stable isotopes of hydrogen (the other being Hydrogen atom, protium, or hydrogen-1). The atomic nucleus, nucleus of a deuterium ato ...
ions. It mainly decays by emission of electrons to 248Cf with a half-life of minutes, but also releases α-particles of 6.87 MeV energy, with the ratio of electrons to α-particles of about 400.
:
The heavier isotopes 249Es, 250Es, 251Es and 252Es were obtained by bombarding 249Bk with α-particles. One to four neutrons are liberated in this process making possible the formation of four different isotopes in one reaction.
:thermal neutron
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium with ...
flux of (2–5)×1014 neutrons·cm−2·s−1 for 500–900 hours:
:Synthesis in nuclear explosions
 The analysis of the debris at the 10- TNT equivalent, megaton ''Ivy Mike'' nuclear test was a part of long-term project. One of the goals of which was studying the efficiency of production of transuranium elements in high-power nuclear explosions. The motivation for these experiments was that synthesis of such elements from uranium requires multiple neutron capture. The probability of such events increases with the
The analysis of the debris at the 10- TNT equivalent, megaton ''Ivy Mike'' nuclear test was a part of long-term project. One of the goals of which was studying the efficiency of production of transuranium elements in high-power nuclear explosions. The motivation for these experiments was that synthesis of such elements from uranium requires multiple neutron capture. The probability of such events increases with the neutron flux
The neutron flux, φ, is a scalar quantity used in nuclear physics and nuclear reactor physics. It is the total length travelled by all free neutrons per unit time and volume. Equivalently, it can be defined as the number of neutrons travelling ...
, and nuclear explosions are the most powerful man-made neutron sources, providing densities of the order 1023 neutrons/cm2 within a microsecond, or about 1029 neutrons/(cm2·s). In comparison, the flux of the HFIR reactor is 5 neutrons/(cm2·s). A dedicated laboratory was set up right at Enewetak Atoll
Enewetak Atoll (; also spelled Eniwetok Atoll or sometimes Eniewetok; mh, Ānewetak, , or , ; known to the Japanese as Brown Atoll or Brown Island; ja, ブラウン環礁) is a large coral atoll of 40 islands in the Pacific Ocean and with it ...
for preliminary analysis of debris, as some isotopes could have decayed by the time the debris samples reached the mainland U.S. The laboratory was receiving samples for analysis as soon as possible, from airplanes equipped with paper filters which flew over the atoll after the tests. Whereas it was hoped to discover new chemical elements heavier than fermium, none of these were found even after a series of megaton explosions conducted between 1954 and 1956 at the atoll.
The atmospheric results were supplemented by the underground test data accumulated in the 1960s at the Nevada Test Site
The Nevada National Security Site (N2S2 or NNSS), known as the Nevada Test Site (NTS) until 2010, is a United States Department of Energy (DOE) reservation located in southeastern Nye County, Nevada, about 65 miles (105 km) northwest of th ...
, as it was hoped that powerful explosions conducted in confined space might result in improved yields and heavier isotopes. Apart from traditional uranium charges, combinations of uranium with americium and thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high me ...
have been tried, as well as a mixed plutonium-neptunium charge, but they were less successful in terms of yield and was attributed to stronger losses of heavy isotopes due to enhanced fission rates in heavy-element charges. Product isolation was problematic as the explosions were spreading debris through melting and vaporizing the surrounding rocks at depths of 300–600 meters. Drilling to such depths to extract the products was both slow and inefficient in terms of collected volumes. #Seaborg, Seaborg, p. 40
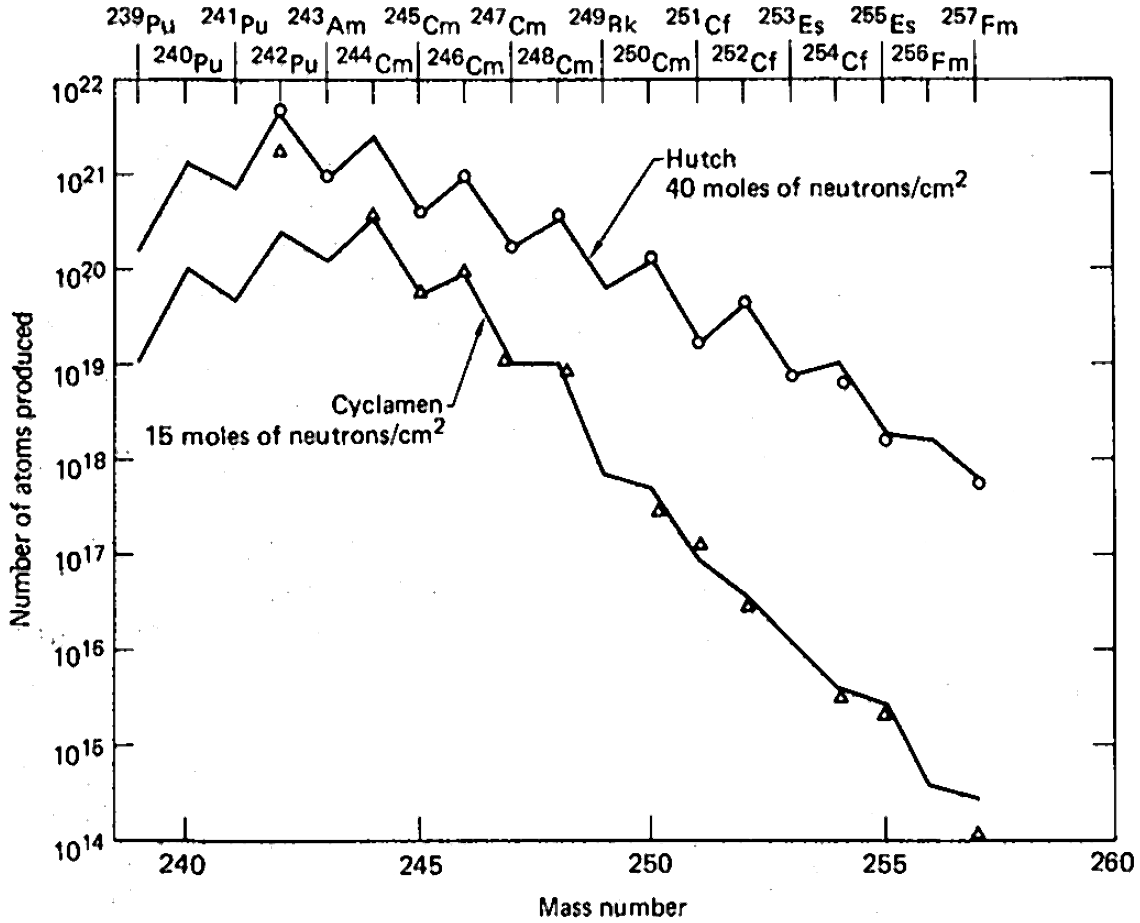
Among the nine underground tests that were carried between 1962 and 1969, the last one was the most powerful and had the highest yield of transuranium elements. Milligrams of einsteinium that would normally take a year of irradiation in a high-power reactor, were produced within a microsecond. However, the major practical problem of the entire proposal was collecting the radioactive debris dispersed by the powerful blast. Aircraft filters adsorbed only about 4 of the total amount, and collection of tons of corals at Enewetak Atoll increased this fraction by only two orders of magnitude. Extraction of about 500 kilograms of underground rocks 60 days after the Hutch explosion recovered only about 1 of the total charge. The amount of transuranium elements in this 500-kg batch was only 30 times higher than in a 0.4 kg rock picked up 7 days after the test which demonstrated the highly non-linear dependence of the transuranium elements yield on the amount of retrieved radioactive rock. #Seaborg, Seaborg, p. 43 Shafts were drilled at the site before the test in order to accelerate sample collection after explosion, so that explosion would expel radioactive material from the epicenter through the shafts and to collecting volumes near the surface. This method was tried in two tests and instantly provided hundreds kilograms of material, but with actinide concentration 3 times lower than in samples obtained after drilling. Whereas such method could have been efficient in scientific studies of short-lived isotopes, it could not improve the overall collection efficiency of the produced actinides. #Seaborg, Seaborg, p. 44
Although no new elements (apart from einsteinium and fermium) could be detected in the nuclear test debris, and the total yields of transuranium elements were disappointingly low, these tests did provide significantly higher amounts of rare heavy isotopes than previously available in laboratories. #Seaborg, Seaborg, p. 47
Separation
 Separation procedure of einsteinium depends on the synthesis method. In the case of light-ion bombardment inside a cyclotron, the heavy ion target is attached to a thin foil, and the generated einsteinium is simply washed off the foil after the irradiation. However, the produced amounts in such experiments are relatively low. Haire, p. 1583 The yields are much higher for reactor irradiation, but there, the product is a mixture of various actinide isotopes, as well as lanthanides produced in the nuclear fission decays. In this case, isolation of einsteinium is a tedious procedure which involves several repeating steps of cation exchange, at elevated temperature and pressure, and chromatography. Separation from berkelium is important, because the most common einsteinium isotope produced in nuclear reactors, 253Es, decays with a half-life of only 20 days to 249Bk, which is fast on the timescale of most experiments. Such separation relies on the fact that berkelium easily oxidizes to the solid +4 state and precipitates, whereas other actinides, including einsteinium, remain in their +3 state in solutions. Haire, pp. 1584–1585
Separation of trivalent actinides from lanthanide fission products can be done by a cation-exchange resin column using a 90% water/10% ethanol solution saturated with
Separation procedure of einsteinium depends on the synthesis method. In the case of light-ion bombardment inside a cyclotron, the heavy ion target is attached to a thin foil, and the generated einsteinium is simply washed off the foil after the irradiation. However, the produced amounts in such experiments are relatively low. Haire, p. 1583 The yields are much higher for reactor irradiation, but there, the product is a mixture of various actinide isotopes, as well as lanthanides produced in the nuclear fission decays. In this case, isolation of einsteinium is a tedious procedure which involves several repeating steps of cation exchange, at elevated temperature and pressure, and chromatography. Separation from berkelium is important, because the most common einsteinium isotope produced in nuclear reactors, 253Es, decays with a half-life of only 20 days to 249Bk, which is fast on the timescale of most experiments. Such separation relies on the fact that berkelium easily oxidizes to the solid +4 state and precipitates, whereas other actinides, including einsteinium, remain in their +3 state in solutions. Haire, pp. 1584–1585
Separation of trivalent actinides from lanthanide fission products can be done by a cation-exchange resin column using a 90% water/10% ethanol solution saturated with hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
(HCl) as eluant
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions.
In a liquid chromatography experiment, for exam ...
. It is usually followed by anion-exchange chromatography using 6 molar HCl as eluant. A cation-exchange resin column (Dowex-50 exchange column) treated with ammonium salts is then used to separate fractions containing elements 99, 100 and 101. These elements can be then identified simply based on their elution position/time, using α-hydroxyisobutyrate solution (α-HIB), for example, as eluant.
Separation of the 3+ actinides can also be achieved by solvent extraction chromatography, using bis-(2-ethylhexyl) phosphoric acid (abbreviated as HDEHP) as the stationary organic phase, and nitric acid as the mobile aqueous phase. The actinide elution sequence is reversed from that of the cation-exchange resin column. The einsteinium separated by this method has the advantage to be free of organic complexing agent, as compared to the separation using a resin column.
Preparation of the metal
Einsteinium is highly reactive and therefore strong reducing agents are required to obtain the pure metal from its compounds. Haire, p. 1588 This can be achieved by reduction of einsteinium(III) fluoride with metalliclithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid el ...
:
:EsF3 + 3 Li → Es + 3 LiF
However, owing to its low melting point and high rate of self-radiation damage, einsteinium has high vapor pressure, which is higher than that of lithium fluoride. This makes this reduction reaction rather inefficient. It was tried in the early preparation attempts and quickly abandoned in favor of reduction of einsteinium(III) oxide with lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lantha ...
metal: Haire, p. 1590
:Es2O3 + 2 La → 2 Es + La2O3
Chemical compounds
Oxides
Einsteinium(III) oxide (Es2O3) was obtained by burning einsteinium(III) nitrate. It forms colorless cubic crystals, which were first characterized from microgram samples sized about 30 nanometers. Greenwood, p. 1268 Two other phases,monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic s ...
and hexagonal, are known for this oxide. The formation of a certain Es2O3 phase depends on the preparation technique and sample history, and there is no clear phase diagram. Interconversions between the three phases can occur spontaneously, as a result of self-irradiation or self-heating. Haire, p. 1598 The hexagonal phase is isotypic with lanthanum oxide
Lanthanum(III) oxide, also known as lanthana, chemical formula , is an inorganic compound containing the rare earth element lanthanum and oxygen. It is used in some ferroelectric materials, as a component of optical materials, and is a feedstock ...
where the Es3+ ion is surrounded by a 6-coordinated group of O2− ions.
Halides
 Einsteinium
Einsteinium halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
s are known for the oxidation states +2 and +3. Holleman, p. 1969 The most stable state is +3 for all halides from fluoride to iodide.
Einsteinium(III) fluoride (EsF3) can be precipitated from einsteinium(III) chloride solutions upon reaction with fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typ ...
ions. An alternative preparation procedure is to exposure einsteinium(III) oxide to chlorine trifluoride
Chlorine trifluoride is an interhalogen compound with the formula ClF3. This colorless, poisonous, corrosive, and extremely reactive gas condenses to a pale-greenish yellow liquid, the form in which it is most often sold (pressurized at room temp ...
(ClF3) or F2 gas at a pressure of 1–2 atmospheres and a temperature between 300 and 400 °C. The EsF3 crystal structure is hexagonal, as in californium(III) fluoride (CfF3) where the Es3+ ions are 8-fold coordinated by fluorine ions in a bicapped trigonal prism
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The oc ...
arrangement. Greenwood, p. 1270
Einsteinium(III) chloride (EsCl3) can be prepared by annealing einsteinium(III) oxide in the atmosphere of dry hydrogen chloride vapors at about 500 °C for some 20 minutes. It crystallizes upon cooling at about 425 °C into an orange solid with a hexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A '' regular hexagon'' has ...
structure of UCl3 type, where einsteinium atoms are 9-fold coordinated by chlorine atoms in a tricapped trigonal prism geometry.Miasoedov, B. F. Analytical chemistry of transplutonium elements, Wiley, 1974 (Original from the University of California), , p. 99 Einsteinium(III) bromide (EsBr3) is a pale-yellow solid with a monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic s ...
structure of AlCl3 type, where the einsteinium atoms are octahedrally coordinated by bromine (coordination number 6).
The divalent compounds of einsteinium are obtained by reducing the trivalent halides with hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
:manuscript draft:2 EsX3 + H2 → 2 EsX2 + 2 HX, X = F, Cl, Br, I Einsteinium(II) chloride (EsCl2), einsteinium(II) bromide (EsBr2), and einsteinium(II) iodide (EsI2) have been produced and characterized by optical absorption, with no structural information available yet. Known oxyhalides of einsteinium include EsOCl, EsOBr and EsOI. These salts are synthesized by treating a trihalide with a vapor mixture of water and the corresponding hydrogen halide: for example, EsCl3 + H2O/HCl to obtain EsOCl. #Seaborg, Seaborg, p. 60
Organoeinsteinium compounds
The high radioactivity of einsteinium has a potential use inradiation therapy
Radiation therapy or radiotherapy, often abbreviated RT, RTx, or XRT, is a therapy using ionizing radiation, generally provided as part of cancer treatment to control or kill malignant cells and normally delivered by a linear accelerator. Radia ...
, and organometallic complexes have been synthesized in order to deliver einsteinium atoms to an appropriate organ in the body. Experiments have been performed on injecting einsteinium citrate
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in t ...
(as well as fermium compounds) to dogs. Einsteinium(III) was also incorporated into beta-diketone chelate
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
complexes, since analogous complexes with lanthanides previously showed strongest UV-excited luminescence
Luminescence is spontaneous emission of light by a substance not resulting from heat; or "cold light".
It is thus a form of cold-body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions or stress on a cryst ...
among metallorganic compounds. When preparing einsteinium complexes, the Es3+ ions were 1000 times diluted with Gd3+ ions. This allowed reducing the radiation damage so that the compounds did not disintegrate during the period of 20 minutes required for the measurements. The resulting luminescence from Es3+ was much too weak to be detected. This was explained by the unfavorable relative energies of the individual constituents of the compound that hindered efficient energy transfer from the chelate matrix to Es3+ ions. Similar conclusion was drawn for other actinides americium, berkelium and fermium.
Luminescence of Es3+ ions was however observed in inorganic hydrochloric acid solutions as well as in organic solution with di(2-ethylhexyl)orthophosphoric acid. It shows a broad peak at about 1064 nanometers (half-width about 100 nm) which can be resonantly excited by green light (ca. 495 nm wavelength). The luminescence has a lifetime of several microseconds and the quantum yield below 0.1%. The relatively high, compared to lanthanides, non-radiative decay rates in Es3+ were associated with the stronger interaction of f-electrons with the inner Es3+ electrons.
Applications
There is almost no use for any isotope of einsteinium outside basic scientific research aiming at production of highertransuranium element
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. ...
s and superheavy element
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, are the chemical elements with atomic number greater than 103. The superheavy elements are those beyond the actinides in the periodic table; the l ...
s.
In 1955, mendelevium
Mendelevium is a synthetic element with the symbol Md ( formerly Mv) and atomic number 101. A metallic radioactive transuranium element in the actinide series, it is the first element by atomic number that currently cannot be produced in macroscopi ...
was synthesized by irradiating a target consisting of about 109 atoms of 253Es in the 60-inch cyclotron at Berkeley Laboratory. The resulting 253Es(α,n)256Md reaction yielded 17 atoms of the new element with the atomic number of 101.
The rare isotope 254Es is favored for production of superheavy element
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, are the chemical elements with atomic number greater than 103. The superheavy elements are those beyond the actinides in the periodic table; the l ...
s because of its large mass, relatively long half-life of 270 days, and availability in significant amounts of several micrograms. Hence 254Es was used as a target in the attempted synthesis of ununennium
Ununennium, also known as eka-francium or element 119, is the hypothetical chemical element with symbol Uue and atomic number 119. ''Ununennium'' and ''Uue'' are the temporary systematic IUPAC name and symbol respectively, which are used until th ...
(element 119) in 1985 by bombarding it with calcium-48
Calcium-48 is a scarce isotope of calcium containing 20 protons and 28 neutrons. It makes up 0.187% of natural calcium by mole fraction. Although it is unusually neutron-rich for such a light nucleus, its beta decay is extremely hindered, and so ...
ions at the superHILAC linear particle accelerator
A linear particle accelerator (often shortened to linac) is a type of particle accelerator that accelerates charged subatomic particles or ions to a high speed by subjecting them to a series of oscillating electric potentials along a linear b ...
at Berkeley, California. No atoms were identified, setting an upper limit for the cross section of this reaction at 300 nanobarns.
:Surveyor 5
Surveyor 5 was the fifth lunar lander of the American uncrewed Surveyor program sent to explore the surface of the Moon. Surveyor 5 landed on Mare Tranquillitatis in 1967. A total of 19,118 images were transmitted to Earth.
Mission
The mission ...
lunar probe. The large mass of this isotope reduced the spectral overlap between signals from the marker and the studied lighter elements of the lunar surface.
Safety
Most of the available einsteinium toxicity data, is from research on animals. Upon ingestion byrats
Rats are various medium-sized, long-tailed rodents. Species of rats are found throughout the order Rodentia, but stereotypical rats are found in the genus ''Rattus''. Other rat genera include ''Neotoma'' (pack rats), ''Bandicota'' (bandicoot ...
, only ~0.01% of it ends in the bloodstream. From there, about 65% goes to the bones, where it would remain for ~50 years if not for its radioactive decay, not to speak of the 3-year maximum lifespan of rats, 25% to the lungs (biological half-life ~20 years, though this is again rendered irrelevant by the short half-life of einsteinium), 0.035% to the testicles or 0.01% to the ovaries – where einsteinium stays indefinitely. About 10% of the ingested amount is excreted. The distribution of einsteinium over bone surfaces is uniform and is similar to that of plutonium.
References
Bibliography
* * * *External links
Einsteinium
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
Age-related factors in radionuclide metabolism and dosimetry: Proceedings
– contains several health related studies of einsteinium {{Authority control Chemical elements Chemical elements with face-centered cubic structure Actinides Synthetic elements Albert Einstein