|
Einsteinium
Einsteinium is a synthetic element with the symbol Es and atomic number 99. Einsteinium is a member of the actinide series and it is the seventh transuranium element. It was named in honor of Albert Einstein. Einsteinium was discovered as a component of the debris of the first hydrogen bomb explosion in 1952. Its most common isotope, einsteinium-253 (half-life 20.47 days), is produced artificially from decay of californium-253 in a few dedicated high-power nuclear reactors with a total yield on the order of one milligram per year. The reactor synthesis is followed by a complex process of separating einsteinium-253 from other actinides and products of their decay. Other isotopes are synthesized in various laboratories, but in much smaller amounts, by bombarding heavy actinide elements with light ions. Owing to the small amounts of produced einsteinium and the short half-life of its most easily produced isotope, there are currently almost no practical applications for it outside ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mendelevium
Mendelevium is a synthetic element with the chemical symbol, symbol Md (#rename, formerly Mv) and atomic number 101. A metallic radioactive transuranium element in the actinide series, it is the first element by atomic number that currently cannot be produced in Macroscopic scale, macroscopic quantities by neutron bombardment of Light element, lighter elements. It is the third-to-last actinide and the ninth transuranic element. It can only be produced in particle accelerators by bombarding lighter elements with charged particles. Seventeen isotopes of mendelevium, isotopes are known; the most stable is 258Md with half-life 51 days; however, the shorter-lived 256Md (half-life 1.17 hours) is most commonly used in chemistry because it can be produced on a larger scale. Mendelevium was discovered by bombarding einsteinium with alpha particles in 1955, the method still used to produce it today. It was named after Dmitri Mendeleev, father of the periodic table of the chemical el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
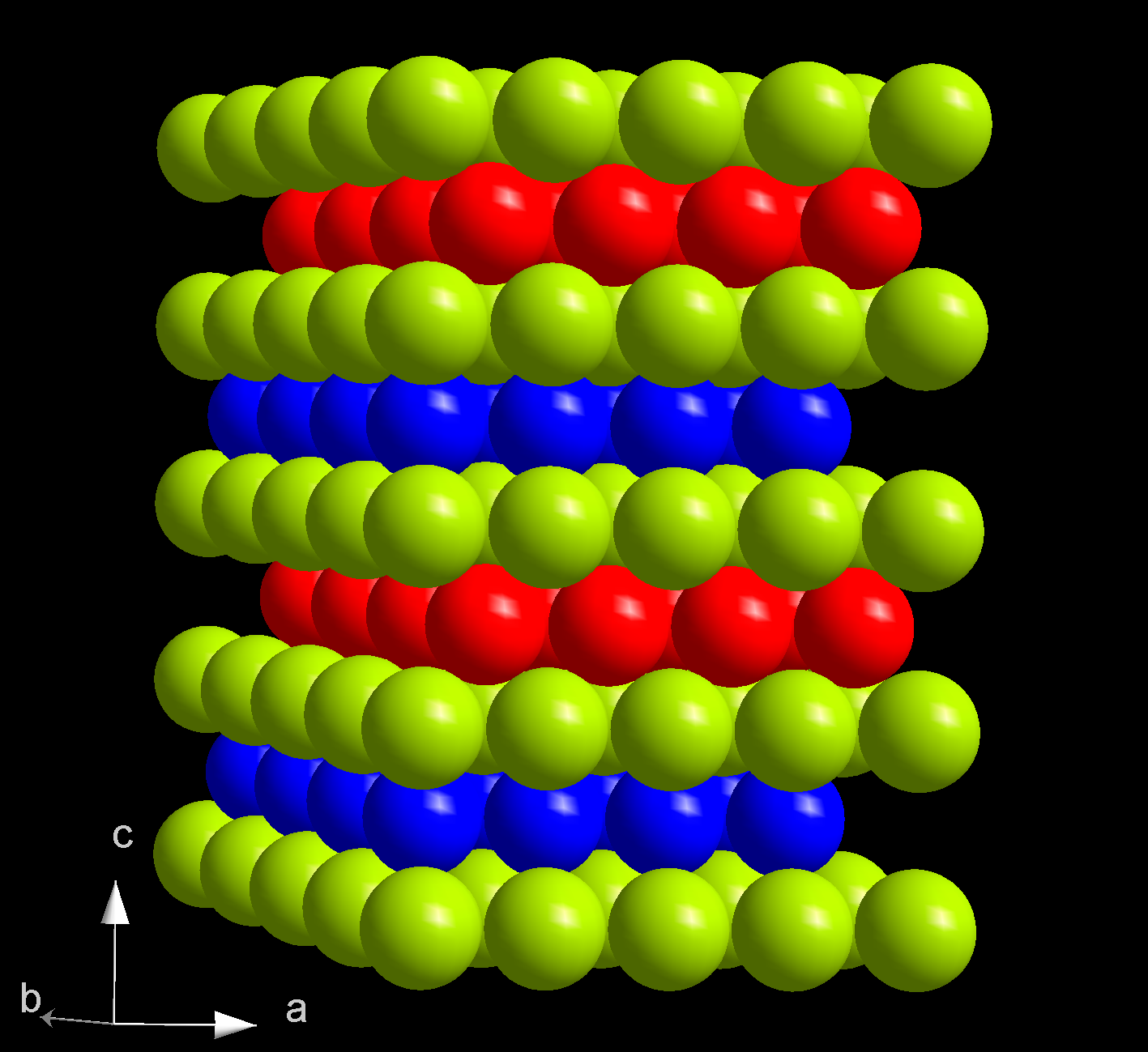
Actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide. The 1985 IUPAC ''Red Book'' recommends that ''actinoid'' be used rather than ''actinide'', since the suffix ''-ide'' normally indicates a negative ion. However, owing to widespread current use, ''actinide'' is still allowed. Since ''actinoid'' literally means ''actinium-like'' (cf. ''humanoid'' or ''android''), it has been argued for semantic reasons that actinium cannot logically be an actinoid, but IUPAC acknowledges its inclusion based on common usage. All the actinides are f-block elements, except the final one (lawrencium) which is a d-block element. Actinium has sometimes been considered d-block instead of lawrencium, but th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berkelium
Berkelium is a transuranic radioactive chemical element with the symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory) where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium. The major isotope of berkelium, 249Bk, is synthesized in minute quantities in dedicated high-flux nuclear reactors, mainly at the Oak Ridge National Laboratory in Tennessee, United States, and at the Research Institute of Atomic Reactors in Dimitrovgrad, Russia. The production of the second-most important isotope, 247Bk, involves the irradiation of the rare isotope 244Cm with high-energy alpha particles. Just over one gram of berkelium has been produced in the United States since 1967. There is no practical a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Symbol
Chemical symbols are the abbreviations used in chemistry for chemical elements, functional groups and chemical compounds. Element symbols for chemical elements normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised. History Earlier symbols for chemical elements stem from classical Latin and Greek vocabulary. For some elements, this is because the material was known in ancient times, while for others, the name is a more recent invention. For example, Pb is the symbol for lead (''plumbum'' in Latin); Hg is the symbol for mercury (''hydrargyrum'' in Greek); and He is the symbol for helium (a new Latin name) because helium was not known in ancient Roman times. Some symbols come from other sources, like W for tungsten (''Wolfram'' in German) which was not known in Roman times. A three-letter temporary symbol may be assigned to a newly synthesized (or not yet synthesized) element. For example, "Uno" was the temporary symbol f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Element
A synthetic element is one of 24 known chemical elements that do not occur naturally on Earth: they have been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; thus, they are called "synthetic", "artificial", or "man-made". The synthetic elements are those with atomic numbers 95–118, as shown in purple on the accompanying periodic table: these 24 elements were first created between 1944 and 2010. The mechanism for the creation of a synthetic element is to force additional protons into the nucleus of an element with an atomic number lower than 95. All synthetic elements are unstable, but they decay at widely varying rates: the half-lives of their longest-lived isotopes range from microseconds to millions of years. Five more elements that were created artificially are strictly speaking not ''synthetic'' because they were later found in nature in trace quantities: 43Tc, 61Pm, 85At, 93Np, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ivy Mike
Ivy Mike was the codename given to the first full-scale test of a thermonuclear device, in which part of the explosive yield comes from nuclear fusion. Ivy Mike was detonated on November 1, 1952, by the United States on the island of Elugelab in Enewetak Atoll, in the now independent island nation of the Marshall Islands, as part of Operation Ivy. It was the first full test of the Teller–Ulam design, a staged fusion device. Due to its physical size and fusion fuel type ( cryogenic liquid deuterium), the "Mike" device was not suitable for use as a deliverable weapon. It was intended as a "technically conservative" proof of concept experiment to validate the concepts used for multi- megaton detonations. As a result of the collection of samples from the explosion by U.S. Air Force pilots, scientists found traces of the isotopes plutonium-246 and plutonium-244, and confirmed the existence of the predicted but undiscovered elements einsteinium and fermium. Schedule Be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding curium with alpha particles ( helium-4 ions). It is an actinide element, the sixth transuranium element to be synthesized, and has the second-highest atomic mass of all elements that have been produced in amounts large enough to see with the naked eye (after einsteinium). The element was named after the university and the U.S. state of California. Two crystalline forms exist for californium at normal pressure: one above and one below . A third form exists at high pressure. Californium slowly tarnishes in air at room temperature. Californium compounds are dominated by the +3 oxidation state. The most stable of californium's twenty known isotopes is californium-251, with a half-life of 898 years. This short half-life means the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argonne National Laboratory
Argonne National Laboratory is a science and engineering research national laboratory operated by UChicago Argonne LLC for the United States Department of Energy. The facility is located in Lemont, Illinois, outside of Chicago, and is the largest national laboratory by size and scope in the Midwest. Argonne had its beginnings in the Metallurgical Laboratory of the University of Chicago, formed in part to carry out Enrico Fermi's work on nuclear reactors for the Manhattan Project during World War II. After the war, it was designated as the first national laboratory in the United States on July 1, 1946. In the post-war era the lab focused primarily on non-weapon related nuclear physics, designing and building the first power-producing nuclear reactors, helping design the reactors used by the United States' nuclear navy, and a wide variety of similar projects. In 1994, the lab's nuclear mission ended, and today it maintains a broad portfolio in basic science research, energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transuranium Element
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. With the exception of neptunium and plutonium (which have been found in trace amounts in nature), all do not occur naturally on Earth and are synthetic. Overview Of the elements with atomic numbers 1 to 92, most can be found in nature, having stable isotopes (such as hydrogen) or very long-lived radioisotopes (such as uranium), or existing as common decay products of the decay of uranium and thorium (such as radon). The exceptions are elements 43, 61, 85, and 87; all four occur in nature, but only in very minor branches of the uranium and thorium decay chains, and thus all save element 87 were first discovered by synthesis in the laboratory rather than in nature (and even element 87 was discovered from purified samples of its parent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Timeline Of Chemical Element Discoveries
The discovery of the 118 chemical elements known to exist as of 2022 is presented in chronological order. The elements are listed generally in the order in which each was first defined as the pure element, as the exact date of discovery of most elements cannot be accurately determined. There are plans to synthesize more elements, and it is not known how many elements are possible. Each element's name, atomic number, year of first report, name of the discoverer, and notes related to the discovery are listed. Periodic table of elements Ancient discoveries Modern discoveries Graphics See also * History of the periodic table * Periodic table * Extended periodic table * ''The Mystery of Matter: Search for the Elements'' (2014/2015 PBS film) * Transfermium Wars References External linksHistory of the Origin of the Chemical Elements and Their DiscoverersLast updated by Boris Pritychenko on March 30, 2004 [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Albert Ghiorso
Albert Ghiorso (July 15, 1915 – December 26, 2010) was an American nuclear scientist and co-discoverer of a record 12 chemical elements on the periodic table. His research career spanned six decades, from the early 1940s to the late 1990s. Biography Early life Ghiorso was born in Vallejo, California on July 15, 1915, of Italian and Spanish ancestry. He grew up in Alameda, California. As a teenager, he built radio circuitry and earned a reputation for establishing radio contacts at distances that outdid the military. He received his BS in electrical engineering from the University of California, Berkeley in 1937. After graduation, he worked for Reginald Tibbets, a prominent amateur radio operator who operated a business supplying radiation detectors to the government. Ghiorso's ability to develop and produce these instruments, as well as a variety of electronic tasks, brought him into contact with the nuclear scientists at the University of California Radiation Laboratory at Be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of California, Berkeley
The University of California, Berkeley (UC Berkeley, Berkeley, Cal, or California) is a public land-grant research university in Berkeley, California. Established in 1868 as the University of California, it is the state's first land-grant university and the founding campus of the University of California system. Its fourteen colleges and schools offer over 350 degree programs and enroll some 31,800 undergraduate and 13,200 graduate students. Berkeley ranks among the world's top universities. A founding member of the Association of American Universities, Berkeley hosts many leading research institutes dedicated to science, engineering, and mathematics. The university founded and maintains close relationships with three national laboratories at Berkeley, Livermore and Los Alamos, and has played a prominent role in many scientific advances, from the Manhattan Project and the discovery of 16 chemical elements to breakthroughs in computer science and genomics. Berkeley i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |