Dicarbonyl on:
[Wikipedia]
[Google]
[Amazon]
 In
In
RCH(OH)CH(OH)R -> RC(O)C(O)R + 2 H2
2,3-Butanedione, 2,3-pentanedione, and 2,3-hexanedione are found in small amounts in various foods. They are used as aroma components in alcohol-free beverages and in baked goods.
CH2(CH3)COC(O)Me -> MeC(O)CH2C(O)Me
1,3-Diketones that can
 Diketones with two methylene groups separating the carbonyl groups, also called γ-diketones, typically coexist with their
Diketones with two methylene groups separating the carbonyl groups, also called γ-diketones, typically coexist with their  This reactivity is the basis of the neurotoxicity of γ-diketones. 1,4-Diketones are also precursor to
This reactivity is the basis of the neurotoxicity of γ-diketones. 1,4-Diketones are also precursor to
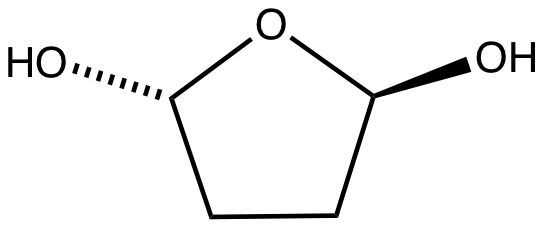 Small aldehydes tend to hydrate. Hydration is prevalent for dialdehydes. Glyoxal forms a series of cyclic hydrates.
Small aldehydes tend to hydrate. Hydration is prevalent for dialdehydes. Glyoxal forms a series of cyclic hydrates.  Similar hydration and cyclization equilibria apply to maleic dialdehyde,
Similar hydration and cyclization equilibria apply to maleic dialdehyde,
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
, a dicarbonyl is a molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
containing two carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containin ...
() groups. Although this term could refer to any organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon- hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and '' functional group'', as well as '' ...
s on each carbonyl, and may also be functionally symmetrical (dialdehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
s, diketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
s, diester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...
s, ''etc.'') or unsymmetrical (keto-esters, keto-acids, ''etc.'').
1,2-Dicarbonyls
1,2-Dialdehyde
The only 1,2-dialdehyde isglyoxal
Glyoxal is an organic compound with the chemical formula OCHCHO. It is the smallest dialdehyde (a compound with two aldehyde groups). It is a crystalline solid, white at low temperatures and yellow near the melting point (15 °C). The liquid ...
, . Like many alkyldialdehydes, glyoxal is encountered almost exclusively as its hydrate and oligomers thereof. These derivatives often behave equivalently to the aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
s since hydration is reversible. Glyoxal condenses readily with amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent su ...
s. Via such reactions, it is a precursor to many heterocycles, e.g. imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non ...
s.
1,2-Diketones
The principal diketone isdiacetyl
Diacetyl (IUPAC systematic name: butanedione or butane-2,3-dione) is an organic compound with the chemical formula (CH3CO)2. It is a yellow liquid with an intensely buttery flavor. It is a vicinal diketone (two C=O groups, side-by-side). Diace ...
, also known as 2,3-butanedione, . 1,2-Diketones are often generated by oxidation (dehydrogenation) of the diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
The most common industrial diol is ...
s:
:Benzil
Benzil (i.e. Bz2, systematically known as 1,2-diphenylethane-1,2-dione) is the organic compound with the formula ( C6H5 CO)2, generally abbreviated ( PhCO)2. This yellow solid is one of the most common diketones. Its main use is as a photoiniti ...
, , is the corresponding diphenyl derivative.
A distinctive feature of 1,2-diketones is the long C-C bond linking the carbonyl groups. This bond distance is about 1.54 Å, compared to 1.45 Å for the corresponding bond in 1,3-butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two viny ...
. The effect is attributed to repulsion between the partial positive charges of the carbonyl carbon atoms.
1,2-Diketones condense with many bifunctional nucleophiles, such as urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important ...
and thiourea
Thiourea () is an organosulfur compound with the formula and the structure . It is structurally similar to urea (), except that the oxygen atom is replaced by a sulfur atom (as implied by the '' thio-'' prefix); however, the properties of ur ...
to give heterocycles. Condensation with aromatic amines gives diketimine
Diimines are organic compounds containing two imine (RCH=NR') groups. Common derivatives are 1,2- diketones and 1,3-diimines. These compounds are used as ligands and as precursors to heterocycles. Diimines are prepared by condensation reactio ...
().
In the cases of 1,2-cyclohexanedione
1,2-Cyclohexanedione is an organic compound with the formula (CH)(CO). It is one of three isomeric cyclohexanediones. It is a colorless compound that is soluble in a variety of organic solvents. It can be prepared by oxidation of cyclohexanone ...
and 1,2-cyclopentanedione
1,2-Cyclopentanedione is the organic compound with the formula (CH2)3(CO)2. It is one of two isomeric cyclopentanediones, the other being 1,3-cyclopentanedione. It was first prepared by base-induced condensation of di
ethylglutarate with diethy ...
, the enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (). The te ...
is about 1-3 kcal/mol more stable than the diketo form.
ortho-Quinone, , is the parent of a large family of 1,2-diketones.
1,2-Diesters and diacids
Oxalic acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early inve ...
and its esters define this family of compounds. The diacid is produced industrially by oxidation of waste sugars. It occurs naturally (as the conjugate base), notably in members of the plant species ''Oxalis''. Condensation of the diesters with diamine
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term ''diamine'' refers mostly to primary diamines, as those are the most reactive.
In terms of quantiti ...
s gives cyclic diamides.
α-Keto- and formylcarboxylic acids
α-Keto-acids and -esters are well known.Pyruvic acid
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic ac ...
() is the parent α-ketoacid. Its conjugate base, pyruvate (), is a component of the citric acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and prote ...
and product of glucose metabolism ( glycolysis). The corresponding aldehyde-acid is glyoxalic acid
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially.
Str ...
().
1,3-Dicarbonyls
1,3-Dialdehydes
The parent 1,3-dialdehyde ismalondialdehyde
Malondialdehyde (MDA) is the organic compound with the nominal formula CH2(CHO)2. A colorless liquid, malondialdehyde is a highly reactive compound that occurs as the enol. It occurs naturally and is a marker for oxidative stress.
Structure and ...
(). Like most dialdehydes, it is rarely encountered as such. Instead it is handled almost exclusively as its hydrate, methyl acetal, and oligomers thereof. These derivatives often behave like the parent. Many 2-substituted derivatives are known. They are often prepared by alkylation of the enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
Bonding and structure
Enolate anions are electr ...
of malondialdehyde.
1,3-Diketones
1,3-Diketones are also called β-diketones. An important member isacetylacetone
Acetylacetone is an organic compound with the chemical formula . It is a colorless liquid, classified as a 1,3- diketone. It exists in equilibrium with a tautomer . These tautomers interconvert so rapidly under most conditions that they are tr ...
, . Dimedone
Dimedone is a cyclic diketone used in organic chemistry to determine whether a compound contains an aldehyde group. Cyclohexanediones in general can be used as catalysts in the formation of transition-metal complexes. Other uses include applica ...
is a cyclic 1,3-diketone. 1,3-Indandione is the cyclic 1,3-diketone fused to a benzene ring. Acetylacetone is prepared industrially by the thermal rearrangement of isopropenylacetate. Another cyclic 1,3-diketone is 2,2,4,4-tetramethylcyclobutanedione, which is a precursor to a useful diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
The most common industrial diol is ...
.
:tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hyd ...
ize to an enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (). The te ...
that is conjugated to the other carbonyl usually exist predominantly in the enol form, and especially when the product can be further stabilized by a six-membered ring containing a hydrogen bond. For example, the percent enol in acetylacetone
Acetylacetone is an organic compound with the chemical formula . It is a colorless liquid, classified as a 1,3- diketone. It exists in equilibrium with a tautomer . These tautomers interconvert so rapidly under most conditions that they are tr ...
, trifluoroacetyacetone, and hexafluoroacetylacetone
Hexafluoroacetylacetone is the chemical compound with the nominal formula CF3C(O)CH2C(O)CF3 (often abbreviated as hfacH). This colourless liquid is a ligand precursor and a reagent used in MOCVD. The compound exists exclusively as the enol CF3C( ...
are 85, 97, and 100%, respectively (neat, 33 °C).
:
Like other diketones, 1,3-diketones are versatile precursors to heterocycles. Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazin ...
, for example, condenses to give pyrazole
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with p''K''b 11.5 ( ...
s.
The conjugate base derived from 1,3-ketones can serve as ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
s to form metal acetylacetonate coordination complexes. In the DeMayo reaction The DeMayo reaction is a photochemical reaction in which the enol of a 1,3-diketone reacts with an alkene (or another species with a C=C bond) and the resulting cyclobutane ring undergoes a retro-aldol reaction to yield a 1,5-diketone:
The net ef ...
1,3-diketones react with alkenes in a photochemical pericyclic reaction
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overlap ...
to form (substituted) 1,5-diketones.
Classically, 1,3-diketones are prepared by the Claisen condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β- diketone. It is named after Ra ...
of a ketone with an ester.
1,3-Diesters and diacids
Malonic acid
Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic aci ...
and its esters are the parent members of this class of dicarbonyls. Also common are the 2-substituted derivatives with the formula , which arise by C-alkylation of the conjugate base (the enolate) .
β-Keto-esters
β-Keto-esters arise readily by the condensation of a pair of esters. A well known example isethyl acetoacetate
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is a colorless liquid. It is widely used as a chemical intermediate in the production of a wide variety of compounds. It is used as a flavoring for food.
Pr ...
(although it is prepared by ethanolysis of ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary monovalent chemical groups (or two separate substitution sites in the same molecule). The name may also refer to the specific compound eth ...
).
1,4-Dicarbonyls
1,4-Dialdehydes
Succinaldehyde
Succinaldehyde or succindialdehyde is an organic compound with the formula (CH2CHO)2. Typical of other dialdehydes, succinaldehyde is highly reactive and is rarely observed as the dialdehyde. Usually, it is handled as the hydrates or methanol-d ...
(CH2CHO)2 is the simplest and parent 1,4-dialdehyde. The aromatic derivative is phthalaldehyde
Phthalaldehyde (sometimes also ''o''-phthalaldehyde or ''ortho''-phthalaldehyde, OPA) is the chemical compound with the formula C6H4(CHO)2. It is one of three isomers of benzene dicarbaldehyde, related to phthalic acid. This pale yellow solid is ...
.
1,4-Diketones
enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (). The te ...
tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hyd ...
s. The preeminent member is acetonylacetone
2,5-Hexanedione (Acetonylacetone) is an aliphatic diketone. It is a colorless liquid. In humans, it is a toxic metabolite of hexane and of 2-hexanone.
Symptoms of poisoning
The chronic toxicity of hexane is attributed to hexane-2,5-dione. T ...
. 1,4-Diketones are useful precursors to heterocycles via the Paal–Knorr synthesis
The Paal–Knorr Synthesis in organic chemistry is a reaction that generates either furans, pyrroles, or thiophenes from 1,4-diketones. It is a synthetically valuable method for obtaining substituted furans and pyrroles, common structural componen ...
, which gives pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-met ...
s:
:furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly ...
s and thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its react ...
s. The condensation of 1,4-diketones (and related substrates) with hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazin ...
s afford dihydropyridazines, which can be converted to pyridazine
Pyridazine is an aromatic, heterocyclic, organic compound with the molecular formula . It contains a six-membered ring with two adjacent nitrogen atoms. It is a colorless liquid with a boiling point of 208 °C. It is isomeric with two othe ...
s.
para-quinone, C4H4(CO)2, is the parent of a large family of 1,4-diketones.
1,4-Diesters and diacids
Succinic acid
Succinic acid () is a dicarboxylic acid with the chemical formula (CH2)2(CO2H)2. The name derives from Latin ''succinum'', meaning amber. In living organisms, succinic acid takes the form of an anion, succinate, which has multiple biological r ...
and its esters are the parent members of this family of 1,4-dicarbonyls. Succinic acid is notable as a component in the citric acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and prote ...
. It forms a cyclic acid anhydride, succinic anhydride
Succinic anhydride, is an organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon- hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms) ...
. Unsaturated members include maleic and fumaric acid
Fumaric acid is an organic compound with the formula HO2CCH=CHCO2H. A white solid, fumaric acid occurs widely in nature. It has a fruit-like taste and has been used as a food additive. Its E number is E297.
The salts and esters are known as fum ...
s and their esters.
1,5-Dicarbonyls
1,5-Dialdehydes
Glutaraldehyde
Glutaraldehyde is an organic compound with the formula . The molecule consists of a five carbon chain doubly terminated with formyl (CHO) groups. It is usually used as a solution in water, and such solutions exists as a collection of hydrates, c ...
(CH2)3(CHO)2 is the simplest and parent 1,5-dialdehyde. It hydrates readily. The aromatic analogue is isophthalaldehyde
Isophthalaldehyde is an organic compound with the formula C6H4(CHO)2. It is one of three isomers of benzene di carbaldehyde, a reduced analog of phthalic acid. It is colorless, although commercial samples often appear yellowish. One preparation ...
.
1,5-Diketones
These diketones have three methylene groups separating the carbonyl groups.1,5-Diesters and diacids
Glutaric acid
Glutaric acid is the organic compound with the formula C3H6(COOH)2 . Although the related "linear" dicarboxylic acids adipic and succinic acids are water-soluble only to a few percent at room temperature, the water-solubility of glutaric acid ...
(CH2)3(CO2H)2 is the parent 1,5-diacid.
Hydration and cyclization
 Small aldehydes tend to hydrate. Hydration is prevalent for dialdehydes. Glyoxal forms a series of cyclic hydrates.
Small aldehydes tend to hydrate. Hydration is prevalent for dialdehydes. Glyoxal forms a series of cyclic hydrates. Succinaldehyde
Succinaldehyde or succindialdehyde is an organic compound with the formula (CH2CHO)2. Typical of other dialdehydes, succinaldehyde is highly reactive and is rarely observed as the dialdehyde. Usually, it is handled as the hydrates or methanol-d ...
hydrates readily to give 2,5-dihydroxytetrahydrofuran. The aromatic phthalaldehyde
Phthalaldehyde (sometimes also ''o''-phthalaldehyde or ''ortho''-phthalaldehyde, OPA) is the chemical compound with the formula C6H4(CHO)2. It is one of three isomers of benzene dicarbaldehyde, related to phthalic acid. This pale yellow solid is ...
also forms hydrated.
 Similar hydration and cyclization equilibria apply to maleic dialdehyde,
Similar hydration and cyclization equilibria apply to maleic dialdehyde, glutaraldehyde
Glutaraldehyde is an organic compound with the formula . The molecule consists of a five carbon chain doubly terminated with formyl (CHO) groups. It is usually used as a solution in water, and such solutions exists as a collection of hydrates, c ...
, and adipaldehyde.
Safety
A number of dicarbonyl compounds are bioactive. Diacetyl is known to cause the lung diseasebronchiolitis obliterans
Bronchiolitis obliterans (BO), also known as obliterative bronchiolitis, constrictive bronchiolitis and popcorn lung, is a disease that results in obstruction of the smallest airways of the lungs ( bronchioles) due to inflammation. Symptoms inclu ...
in those individuals exposed to it in an occupational setting. Dialdehydes, e.g. glutaraldehyde and malonaldehyde, are fixatives or sterilizers.
See also
* TriketoneReferences
{{Reflist