|
Keto-acid
In organic chemistry, keto acids or ketoacids (also called oxo acids or oxoacids) are organic compounds that contain a carboxylic acid group () and a ketone group ().Franz Dietrich Klingler, Wolfgang Ebertz "Oxocarboxylic Acids" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. In several cases, the keto group is hydrated. The alpha-keto acids are especially important in biology as they are involved in the Krebs citric acid cycle and in glycolysis. Common types of keto acids include: *Alpha-keto acids, alpha-ketoacids, or 2-oxoacids have the keto group adjacent to the carboxylic acid. They often arise by oxidative deamination of amino acids, and reciprocally, they are precursors to the same. Alpha-keto acids possesses extensive chemistry as acylation agents. Furthermore, alpha-keto acids such as phenylpyruvic acid are endogenous sources for carbon monoxide (as a gasotransmitter) and pharmaceutical prodrug scaffold. Important representatives: **pyru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyruvic Acid
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell. Pyruvic acid can be made from glucose through glycolysis, converted back to carbohydrates (such as glucose) via gluconeogenesis, or to fatty acids through a reaction with acetyl-CoA. It can also be used to construct the amino acid alanine and can be converted into ethanol or lactic acid via fermentation. Pyruvic acid supplies energy to cells through the citric acid cycle (also known as the Krebs cycle) when oxygen is present (aerobic respiration), and alternatively ferments to produce lactate when oxygen is lacking. Chemistry In 1834, Théophile-Jules Pelouze distilled tartaric acid and isolated glutaric acid and another unknown organic acid. Jöns Jacob Berzelius characterized this other acid the following year and named pyruvic acid because it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Krebs Citric Acid Cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism and may have originated abiogenically. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized. The name of this metabolic pathway is derived from the citric acid (a tricarboxy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citric Acid Cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism and may have originated abiogenically. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized. The name of this metabolic pathway is derived from the citric acid (a tricarboxy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetoacetic Acid
Acetoacetic acid (also acetoacetate and diacetic acid) is the organic compound with the formula CHCOCHCOOH. It is the simplest beta-keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite stable, are produced on a large scale industrially as precursors to dyes. Acetoacetic acid is a weak acid. Biochemistry Under typical physiological conditions, acetoacetic acid exists as its conjugate base, acetoacetate. Acetoacetate is produced in the mitochondria of the liver from acetoacetyl coenzyme A (CoA). First, another acetyl group is added from acetyl CoA to form 3-hydroxy-3-methylglutaryl CoA, then an acetyl CoA is lost from this, yielding acetoacetate. The initial acetoacetate can come from the last cycle in the beta oxidation of a fatty acid, or it can be synthesized from two acetyl CoA molecules, catalyzed by thiolase. In mammals, acetoacetate produced in the liver (along with the other two " ketone bodies") is released into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamic Acid
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons. Its molecular formula is . Glutamic acid exists in three optically isomeric forms; the dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxyl groups −COOH and one amino group −. However, in the solid state and mildly acidic water solutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
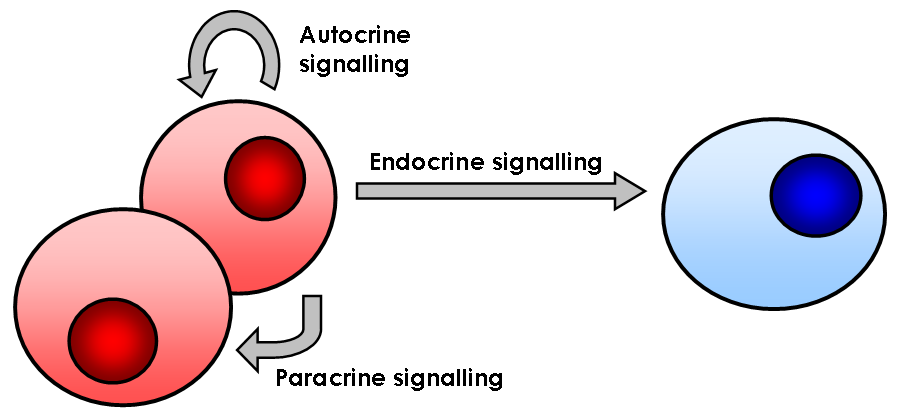
Cell Signaling
In biology, cell signaling (cell signalling in British English) or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. Cell signaling is a fundamental property of all cellular life in prokaryotes and eukaryotes. Signals that originate from outside a cell (or extracellular signals) can be physical agents like mechanical pressure, voltage, temperature, light, or chemical signals (e.g., small molecules, peptides, or gas). Cell signaling can occur over short or long distances, and as a result can be classified as autocrine, juxtacrine, intracrine, paracrine, or endocrine. Signaling molecules can be synthesized from various biosynthetic pathways and released through passive or active transports, or even from cell damage. Receptors play a key role in cell signaling as they are able to detect chemical signals or physical stimuli. Receptors are generally proteins located on the cell surface or within the interio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coenzyme
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from ligands in that they often derive their function by remaining bound. Cofactors can be divided into two types: inorganic ions and complex organic molecules called coenzymes. Coenzymes are mostly derived from vitamins and other organic essential nutrients in small amounts. (Note that some scientists limit the use of the term "cofactor" for inorganic substances; both types are included here.) Coenzymes are further divided into two types. The first is called a "prosthetic group", which consists of a coenzyme that is tightly (or even covalently) and permanently bound to a protein. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transamination
Transamination is a chemical reaction that transfers an amino group to a ketoacid to form new amino acids. This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential amino acids to non-essential amino acids (amino acids that can be synthesized de novo by the organism). Transamination in biochemistry is accomplished by enzymes called transaminases or aminotransferases. α-ketoglutarate acts as the predominant amino-group acceptor and produces glutamate as the new amino acid. :Aminoacid + α-ketoglutarate ↔ α-keto acid + glutamate Glutamate's amino group, in turn, is transferred to oxaloacetate in a second transamination reaction yielding aspartate. :Glutamate + oxaloacetate ↔ α-ketoglutarate + aspartate Mechanism of action Transamination catalyzed by aminotransferase occurs in two stages. In the first step, the α amino group of an amino acid is transferred to the enzyme, producing the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases (Enzyme Commission number, EC number 4.1.1). In organic chemistry The term "decarboxylation" usually means replacement of a carboxyl group () with a hydrogen atom: :RCO2H -> RH + CO2 Decarboxylation is one of the oldest known organic reactions. It is one of the processes assumed to accompany pyrolysis and destructive distillation. Metal salts, especially copper compounds, facilitate the reaction via the intermediacy of metal carboxylate complexes. Decarboxylation of aryl carboxylates can generate the equivalent of the correspond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Claisen Condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887. Requirements At least one of the reagents must be enolizable (have an α-proton and be able to undergo deprotonation to form the enolate anion). There are a number of different combinations of enolizable and nonenolizable carbonyl compounds that form a few different types of Claisen. The base used must not interfere with the reaction by undergoing nucleophilic substitution or addition with a carbonyl carbon. For this reason, the conjugate sodium alkoxide base of the alcohol formed (e.g. sodium ethoxide if ethanol is formed) is often used, since the alkoxide is regenerated. In mixed Claisen condensations, a non-nucleophilic base such as lithium diisopropylamide, or L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Levulinic Acid
Levulinic acid, or 4-oxopentanoic acid, is an organic compound with the formula CH3C(O)CH2CH2CO2H. It is classified as a keto acid. This white crystalline solid is soluble in water and polar organic solvents. It is derived from degradation of cellulose and is a potential precursor to biofuels, such as ethyl levulinate. Synthesis Levulinic acid was first prepared in 1840 by Dutch chemist Gerardus Johannes Mulder by heating fructose with hydrochloric acid. The first commercial production of levulinic acid began as a batchwise process in an autoclave by starch manufacturer A. E. Staley in the 1940s. In 1953 Quaker Oats developed a continuous process for the production of levulinic acid. In 1956 it was identified as a platform chemical with high potential. and in 2004 the US Department of Energy (U.S. DoE) identified levulinic acid as one of the 12 potential platform chemicals in the biorefinery concept. The synthesis of levulinic acid from hexoses (glucose, fructose) or starch in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |