Coproporphyrin on:
[Wikipedia]
[Google]
[Amazon]
 Porphyrins ( ) are a group of heterocyclic macrocycle organic compounds, composed of four modified
Porphyrins ( ) are a group of heterocyclic macrocycle organic compounds, composed of four modified
File:PPIXtransH.png, Derivatives of protoporphyrin IX are common in nature, the precursor to hemes.
File:H2octaethylporphyrin.png , Octaethylporphyrin (H2OEP) is a synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, OEP2− is highly symmetrical.
File:H2TPP.png, Tetraphenylporphyrin (H2TPP)is another synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, TPP2− is highly symmetrical. Another difference is that its methyne centers are occupied by phenyl groups.
File:Heme b.svg, Simplified view of heme, a complex of a protoporphyrin IX.

 Porphyrins are often used to construct structures in supramolecular chemistry. These systems take advantage of the Lewis acidity of the metal, typically zinc. An example of a host–guest complex that was constructed from a macrocycle composed of four porphyrins. A guest-free base porphyrin is bound to the center by coordination with its four-pyridine substituents.
Porphyrins are often used to construct structures in supramolecular chemistry. These systems take advantage of the Lewis acidity of the metal, typically zinc. An example of a host–guest complex that was constructed from a macrocycle composed of four porphyrins. A guest-free base porphyrin is bound to the center by coordination with its four-pyridine substituents.
File:H2TPP.png, Lewis structure for ''meso''-tetraphenylporphyrin
File:Meso-tetraphenylporphyrin UV-vis.JPG, UV–vis readout for ''meso''-tetraphenylporphyrin
File:Porfirina activada con la luz.svg, Light-activated porphyrin. Monatomic oxygen. Cellular aging
Journal of Porphyrins and Phthalocyanines
Porphynet – an informative site about porphyrins and related structures
{{Authority control Biomolecules Metabolism Photosynthetic pigments Chelating agents
pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-meth ...
subunits interconnected at their α carbon atoms via methine bridges (=CH−). The parent of porphyrin is porphine, a rare chemical compound of exclusively theoretical interest. Substituted porphines are called porphyrins. With a total of 26 π-electrons, of which 18 π-electrons form a planar, continuous cycle, the porphyrin ring structure is often described as aromatic. One result of the large conjugated system
In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as ...
is that porphyrins typically absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored. The name "porphyrin" derives from the Greek word πορφύρα (''porphyra''), meaning ''purple''.
Complexes of porphyrins
Concomitant with the displacement of two N-''H'' protons, porphyrins bind metal ions in the N4 "pocket". The metal ion usually has a charge of 2+ or 3+. A schematic equation for these syntheses is shown: :H2porphyrin + Lnsup>2+ → M(porphyrinate)Ln−4 + 4 L + 2 H+, where M = metal ion and L = a ligand The insertion of the metal center is slow in the absence of catalysts. In nature, these catalysts (enzymes) are called chelatases. When there is no metal ion (or atom) bound to the nitrogens in the center, then the compounds are called ''free porphine'' or ''free porphyrin''. If they are bonded to a metal in the center, then they are ''bound''. A porphyrin with an iron atom of the type found inmyoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglobi ...
, hemoglobin, or certain cytochromes is called heme.
Metal complexes derived from porphyrins, often called metalloporphyins, occur naturally. One of the best-known families of porphyrin complexes is heme, the pigment in red blood cells, a cofactor of the protein hemoglobin. Porphin is the simplest porphyrin, a rare compound of theoretical interest.
Ancient porphyrins
A geoporphyrin, also known as a petroporphyrin, is a porphyrin of geologic origin. They can occur incrude oil
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crude ...
, oil shale, coal, or sedimentary rocks. Abelsonite is possibly the only geoporphyrin mineral, as it is rare for porphyrins to occur in isolation and form crystals.
The field of organic geochemistry had its origins in the isolation of porphyrins from petroleum. This finding helped establish the biological origins of petroleum. Petroleum is sometimes "fingerprinted" by analysis of trace amounts of nickel and vanadyl porphyrins.
Biosynthesis
In non-photosyntheticeukaryotes
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
such as animals, insects, fungi, and protozoa
Protozoa (singular: protozoan or protozoon; alternative plural: protozoans) are a group of single-celled eukaryotes, either free-living or parasitic, that feed on organic matter such as other microorganisms or organic tissues and debris. Histo ...
, as well as the α-proteobacteria group of bacteria, the committed step
In enzymology, the committed step (also known as the ''first'' committed step) is an effectively irreversible enzymatic reaction that occurs at a branch point during the biosynthesis of some molecules.
As the name implies, after this step, the mol ...
for porphyrin biosynthesis
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. ...
is the formation of δ-aminolevulinic acid (δ-ALA, 5-ALA or dALA) by the reaction of the amino acid glycine with succinyl-CoA from the citric acid cycle. In plants, algae
Algae (; singular alga ) is an informal term for a large and diverse group of photosynthetic eukaryotic organisms. It is a polyphyletic grouping that includes species from multiple distinct clades. Included organisms range from unicellular mic ...
, bacteria (except for the α-proteobacteria group) and archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebac ...
, it is produced from glutamic acid
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synt ...
via glutamyl-tRNA and glutamate-1-semialdehyde. The enzymes involved in this pathway are glutamyl-tRNA synthetase, glutamyl-tRNA reductase, and glutamate-1-semialdehyde 2,1-aminomutase. This pathway is known as the C5 or Beale pathway.
Two molecules of dALA are then combined by porphobilinogen synthase to give porphobilinogen (PBG), which contains a pyrrole ring. Four PBGs are then combined through deamination into hydroxymethyl bilane (HMB), which is hydrolysed to form the circular tetrapyrrole uroporphyrinogen III. This molecule undergoes a number of further modifications. Intermediates are used in different species to form particular substances, but, in humans, the main end-product protoporphyrin IX is combined with iron to form heme. Bile pigments are the breakdown products of heme.
The following scheme summarizes the biosynthesis of porphyrins, with references by EC number and the OMIM database. The porphyria associated with the deficiency of each enzyme is also shown:
Laboratory synthesis
A common synthesis for porphyrins is theRothemund reaction
The Rothemund reaction is a condensation/oxidation process that converts four pyrroles and four aldehydes into a porphyrin. It is based on work by Paul Rothemund, who first reported it in 1936. The method underpin more modern synthesis such as tho ...
, first reported in 1936, which is also the basis for more recent methods described by Adler and Longo. The general scheme is a condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to ...
and oxidation process starting with pyrrole and an aldehyde.
:
Applications
Photodynamic therapy
Porphyrins have been evaluated in the context of photodynamic therapy (PDT) since they strongly absorb light, which is then converted to heat in the illuminated areas. This technique has been applied in macular degeneration usingverteporfin
Verteporfin (trade name Visudyne), a benzoporphyrin derivative, is a medication used as a photosensitizer for photodynamic therapy to eliminate the abnormal blood vessels in the eye associated with conditions such as the wet form of macular degen ...
.
PDT is considered a noninvasive cancer treatment, involving the interaction between light of a determined frequency, a photo-sensitizer, and oxygen. This interaction produces the formation of a highly reactive oxygen species (ROS), usually singlet oxygen, as well as superoxide anion, free hydroxyl radical, or hydrogen peroxide. These high reactive oxygen species react with susceptible cellular organic biomolecules such as; lipids, aromatic amino acids, and nucleic acid heterocyclic bases, to produce oxidative radicals that damage the cell, possibly inducing apoptosis or even necrosis.
Toxicology
Heme biosynthesis is used as biomarker in environmental toxicology studies. While excess production of porphyrins indicate organochlorine exposure, lead inhibitsALA dehydratase
Aminolevulinic acid dehydratase (porphobilinogen synthase, or ALA dehydratase, or aminolevulinate dehydratase) is an enzyme () that in humans is encoded by the ''ALAD'' gene. Porphobilinogen synthase (or ALA dehydratase, or aminolevulinate de ...
enzyme.
Biological applications
Porphyrins have been investigated as possible anti-inflammatory agents and evaluated on their anti-cancer and anti-oxidant activity. Several porphyrin-peptide conjugates were found to have antiviral activity against HIV ''in vitro''.Potential applications
Biomimetic catalysis
Although not commercialized, metalloporphyrin complexes are widely studied as catalysts for the oxidation of organic compounds. Particularly popular for such laboratory research are complexes of ''meso''- tetraphenylporphyrin and octaethylporphyrin. Complexes with Mn, Fe, and Co catalyze a variety of reactions of potential interest inorganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. Some complexes emulate the action of various heme enzymes such as cytochrome P450
Cytochromes P450 (CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are ...
, lignin peroxidase
In enzymology, a lignin peroxidase () is an enzyme that catalyzes the chemical reaction
:1,2-bis(3,4-dimethoxyphenyl)propane-1,3-diol + H2O2 \rightleftharpoons 3,4-dimethoxybenzaldehyde + 1-(3,4-dimethoxyphenyl)ethane-1,2-diol + H2O
Thus, the ...
. Metalloporphyrins are also studied as catalysts for water splitting, with the purpose of generating molecular hydrogen and oxygen for fuel cells.
Molecular electronics and sensors
Porphyrin-based compounds are of interest as possible components of molecular electronics and photonics. Synthetic porphyrin dyes have been incorporated in prototype dye-sensitized solar cells. Metalloporphyrins have been investigated as sensors. Phthalocyanines, which are structurally related to porphyrins, are used in commerce as dyes and catalysts, but porphyrins are not.Supramolecular chemistry
 Porphyrins are often used to construct structures in supramolecular chemistry. These systems take advantage of the Lewis acidity of the metal, typically zinc. An example of a host–guest complex that was constructed from a macrocycle composed of four porphyrins. A guest-free base porphyrin is bound to the center by coordination with its four-pyridine substituents.
Porphyrins are often used to construct structures in supramolecular chemistry. These systems take advantage of the Lewis acidity of the metal, typically zinc. An example of a host–guest complex that was constructed from a macrocycle composed of four porphyrins. A guest-free base porphyrin is bound to the center by coordination with its four-pyridine substituents.
Theoretical interest in aromaticity
Porphyrinoid macrocycles can show variable aromaticity. An Hückel aromatic porphyrin is porphycene. antiaromatic, Mobius aromatic, and non aromatic porphyrinoid macrocycles are known.See also
* A porphyrin-related disease: porphyria * Porphyrin coordinated to iron: heme * A heme-containing group of enzymes:Cytochrome P450
Cytochromes P450 (CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are ...
* Porphyrin coordinated to magnesium: chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to a ...
* The one-carbon-shorter analogues: corroles, including vitamin B12, which is coordinated to a cobalt
* Corphins, the highly reduced porphyrin coordinated to nickel that binds the Cofactor F430 active site in methyl coenzyme M reductase
In enzymology, coenzyme-B sulfoethylthiotransferase, also known as methyl-coenzyme M reductase (MCR) or most systematically as 2-(methylthio)ethanesulfonate:N-(7-thioheptanoyl)-3-O-phosphothreonine S-(2-sulfoethyl)thiotransferase is an enzyme that ...
(MCR)
* Nitrogen-substituted porphyrins: phthalocyanine
Gallery
Related species
In nature
Several heterocycles related to porphyrins are found in nature, almost always bound to metal ions. These includeSynthetic
A benzoporphyrin is a porphyrin with a benzene ring fused to one of the pyrrole units. e.g.verteporfin
Verteporfin (trade name Visudyne), a benzoporphyrin derivative, is a medication used as a photosensitizer for photodynamic therapy to eliminate the abnormal blood vessels in the eye associated with conditions such as the wet form of macular degen ...
is a benzoporphyrin derivative.
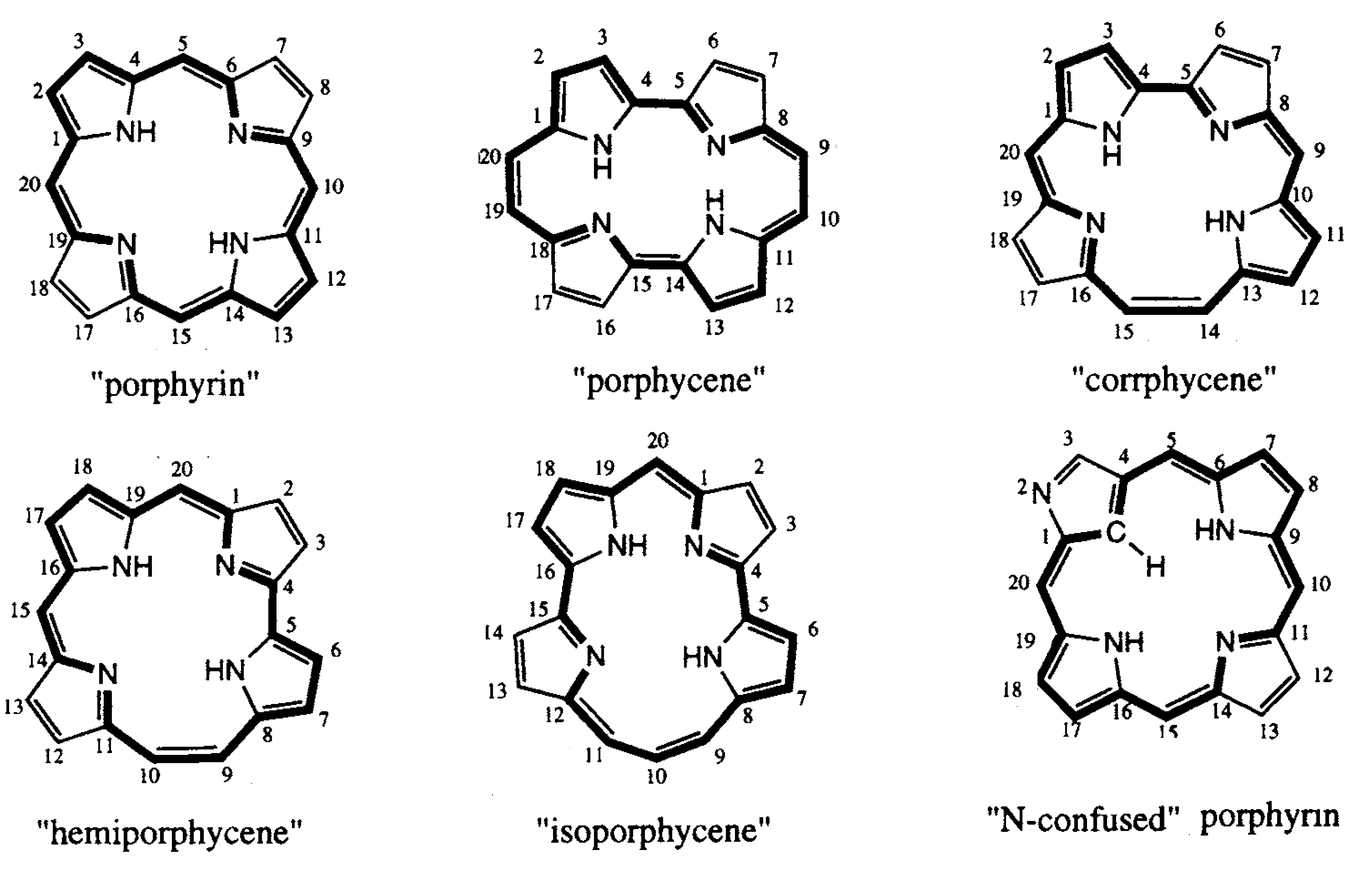
Non-natural porphyrin isomers
The first synthetic porphyrinisomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
was reported by Emanual Vogel and coworkers in 1986. This isomer 8orphyrin-(2.0.2.0) is named as porphycene, and the central N4 Cavity forms a rectangle
In Euclidean plane geometry, a rectangle is a quadrilateral with four right angles. It can also be defined as: an equiangular quadrilateral, since equiangular means that all of its angles are equal (360°/4 = 90°); or a parallelogram containi ...
shape as shown in figure. Porphycenes showed interesting photophysical behavior and found versatile compound towards the photodynamic therapy. This inspired Vogel and Sessler to took up the challenge of preparing 8orphyrin-(2.1.0.1) and named it as Corrphycene or Porphycerin. The third porphyrin that is 8orphyrin-(2.1.1.0), was reported by Callot and Vogel-Sessler. Vogel and coworkers reported successful isolation of 8orphyrin-(3.0.1.0) or Isoporphycene. The Japanese scientist Furuta and Polish scientist Latos-Grażyński almost simultaneously reported the N-Confused porphyrins. The inversion of one of the pyrrolic subunits in the macrocyclic ring resulted to face one of the nitrogen atom outside of the core of the macrocycle.

References
External links
Journal of Porphyrins and Phthalocyanines
Porphynet – an informative site about porphyrins and related structures
{{Authority control Biomolecules Metabolism Photosynthetic pigments Chelating agents