|
Uroporphyrinogen III
Uroporphyrinogen III is a tetrapyrrole, the first macrocyclic intermediate in the biosynthesis of heme, chlorophyll, vitamin B12, and siroheme. It is a colorless compound, like other porphyrinogens. Structure The molecular structure of uroporphyrinogen III can be described as a hexahydroporphine core, where each pyrrole ring has the hydrogen atoms on its two outermost carbons replaced by an acetic acid group (, "A") and a propionic acid group (, "P"). The groups are attached in an asymmetric way: going around the macrocycle, the order is AP-AP-AP-PA. Biosynthesis and metabolism In the general porphyrin biosynthesis pathway, uroporphyrinogen III is derived from the linear tetrapyrrole preuroporphyrinogen (a substituted hydroxymethylbilane) by the action of the enzyme uroporphyrinogen-III cosynthase. The conversion entails a reversal of the last pyrrole unit (thus swapping the acetic and propionic acid groups) and a condensation reaction that closes the macrocycle by eliminati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen III
Uroporphyrinogen III is a tetrapyrrole, the first macrocyclic intermediate in the biosynthesis of heme, chlorophyll, vitamin B12, and siroheme. It is a colorless compound, like other porphyrinogens. Structure The molecular structure of uroporphyrinogen III can be described as a hexahydroporphine core, where each pyrrole ring has the hydrogen atoms on its two outermost carbons replaced by an acetic acid group (, "A") and a propionic acid group (, "P"). The groups are attached in an asymmetric way: going around the macrocycle, the order is AP-AP-AP-PA. Biosynthesis and metabolism In the general porphyrin biosynthesis pathway, uroporphyrinogen III is derived from the linear tetrapyrrole preuroporphyrinogen (a substituted hydroxymethylbilane) by the action of the enzyme uroporphyrinogen-III cosynthase. The conversion entails a reversal of the last pyrrole unit (thus swapping the acetic and propionic acid groups) and a condensation reaction that closes the macrocycle by eliminati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrapyrrole
Tetrapyrroles are a class of chemical compounds that contain four pyrrole or pyrrole-like rings. The pyrrole/pyrrole derivatives are linked by ( =- or -- units), in either a linear or a cyclic fashion. Pyrroles are a five-atom ring with four carbon atoms and one nitrogen atom. Tetrapyrroles are common cofactors in biochemistry and their biosynthesis and degradation feature prominently in the chemistry of life. Some tetrapyrroles form the active core of compounds with crucial biochemical roles in living systems, such as hemoglobin and chlorophyll. In these two molecules, in particular, the pyrrole macrocycle ring frames a metal atom, that forms a coordination compound with the pyrroles and plays a central role in the biochemical function of those molecules. Structure Linear tetrapyrroles (called bilanes) include: *Heme breakdown products (e.g., bilirubin, biliverdin) * Phycobilins (found in cyanobacteria) *Luciferins as found in dinoflagellates and euphausiid shrimps (krill) F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
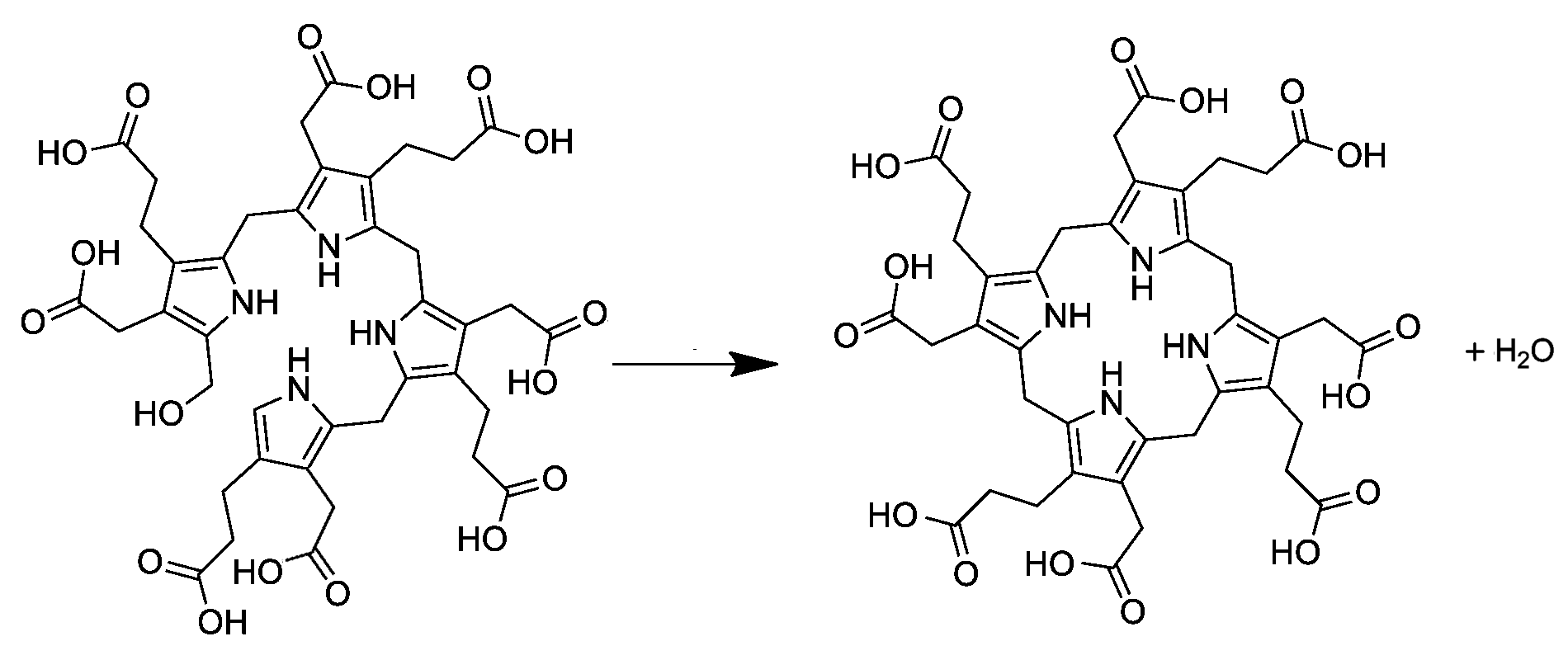
Hydroxymethylbilane
Hydroxymethylbilane, also known as preuroporphyrinogen, is an organic compound that occurs in living organisms during the synthesis of porphyrins, a group of critical substances that include haemoglobin, myoglobin, and chlorophyll. The name is often abbreviated as HMB. The compound is a substituted bilane, a chain of four pyrrole rings interconnected by methylene bridges . The chain starts with a hydroxymethyl group and ends with an hydrogen, in place of the respective methylene bridges. The other two carbon atoms of each pyrrole cycle are connected to an acetic acid group and a propionic acid group , in that order. The compound is generated from four molecules of porphobilinogen by the enzyme porphobilinogen deaminase: The enzyme uroporphyrinogen III synthase closes the chain to form a porphyrinogen a class of compounds with the hexahydroporphine macrocycle; specifically, uroporphyrinogen III. In the absence of the enzyme, the compound undergoes spontaneous cyclization a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen I
Uroporphyrinogen I is an isomer of uroporphyrinogen III, a metabolic intermediate in the biosynthesis of heme. A type of porphyria is caused by production of uroporphyrinogen I instead of III. Biosynthesis and metabolism In living organisms, uroporphyrinogen I occurs as a side branch of the main porphyrin synthesis pathway. In the normal pathway, the linear tetrapyrrole precursor preuroporphyrinogen (a substituted hydroxymethylbilane) is converted by the enzyme uroporphyrinogen-III cosynthase into the cyclic uroporphyrinogen III; which is then converted to coproporphyrinogen III on the way to porphyrins like heme. Uroporphyrinogen I is instead produced spontaneously from preuroporphyrinogen when the enzyme is not present. The difference between the I and III forms is the arrangement of the four carboxyethyl groups (propionic acid, "P") and the four carboxymethyl groups (acetic acid, "A"). The non-enzymatic conversion to uroporphyrinogen I results in the sequence AP-AP-AP-AP ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Isomer
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a chemical compound, compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct chemical bond, bonds between them. The term metamer was formerly used for the same concept. For example, butanol , methyl propyl ether , and diethyl ether have the same molecular formula but are three distinct structural isomers. The concept applies also to polyatomic ions with the same total charge. A classical example is the cyanate ion and the fulminate ion . It is also extended to ionic compounds, so that (for example) ammonium cyanate and urea are considered structural isomers,William F. Bynum, E. Janet Browne, Roy Porter (2014): ''Dictionary of the History of Science''. 530 pages. and so are methylammonium formate and ammonium acetate . Structural isomerism is the most radical type of isomerism. It is opposed to stereoisomerism, in which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sirohydrochlorin
Sirohydrochlorin is a tetrapyrrole macrocyclic metabolic intermediate in the biosynthesis of sirohaem, the iron-containing prosthetic group in sulfite reductase enzymes. It is also the biosynthetic precursor to cofactor F430, an enzyme which catalyzes the release of methane in the final step of methanogenesis. Structure Sirohydrochlorin was first isolated in the early 1970s when it was shown to be the metal-free form of the prosthetic group in the ferredoxin-nitrite reductase from spinach. Its chemical identity was established by spectroscopy and by total synthesis. Biosynthesis Sirohydrochlorin is derived from a tetrapyrrolic structural framework created by the enzymes deaminase and cosynthetase which transform aminolevulinic acid via porphobilinogen and hydroxymethylbilane to uroporphyrinogen III. The latter is the first macrocyclic intermediate common to haem, chlorophyll, sirohaem and vitamin B12. Uroporphyrinogen III is subsequently transformed by the addition of two meth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrosirohydrochlorin
Dihydrosirohydrochlorin is one of several naturally occurring tetrapyrrole macrocyclic metabolic intermediates in the biosynthesis of vitamin B12 (cobalamin). Its oxidised form, sirohydrochlorin, is precursor to sirohaem, the iron-containing prosthetic group in sulfite reductase enzymes. Further biosynthetic transformations convert sirohydrochlorin to cofactor F430 for an enzyme which catalyzes the release of methane in the final step of methanogenesis. Biosynthesis Dihydrosirohydrochlorin is derived from a tetrapyrrolic structural framework created by the enzymes deaminase and cosynthetase which transform aminolevulinic acid via porphobilinogen and hydroxymethylbilane to uroporphyrinogen III. The latter is the first macrocyclic intermediate common to haem, chlorophyll, sirohaem and vitamin B12. Uroporphyrinogen III is subsequently transformed by the addition of two methyl groups to form dihydrosirohydrochlorin. See also *Cobalamin biosynthesis *Sirohydrochlorin *Precorrin-2 dehy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen III Decarboxylase
Uroporphyrinogen III decarboxylase (uroporphyrinogen decarboxylase, or UROD) is an enzyme () that in humans is encoded by the ''UROD'' gene. Function Uroporphyrinogen III decarboxylase is a homodimeric enzyme () that catalyzes the fifth step in heme biosynthesis, which corresponds to the elimination of carboxyl groups from the four acetate side chains of uroporphyrinogen III to yield coproporphyrinogen III: :uroporphyrinogen III \rightleftharpoons coproporphyrinogen III + 4 CO2 Clinical significance Mutations and deficiency in this enzyme are known to cause familial porphyria cutanea tarda Porphyria cutanea tarda is the most common subtype of porphyria. The disease is named because it is a porphyria that often presents with skin manifestations later in life. The disorder results from low levels of the enzyme responsible for the fift ... and hepatoerythropoietic porphyria. At least 65 disease-causing mutations in this gene have been discovered. Mechanism At low substr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coproporphyrinogen III
Coproporphyrinogen III is a metabolic intermediate in the biosynthesis of many compounds that are critical for living organisms, such as hemoglobin and chlorophyll. It is a colorless solid. The compound is a porphyrinogen, a class of compounds characterized by a hexahydroporphine core with various side chains. The coproporphyrinogens have the outermost hydrogen atoms of the core replaced by four methyl groups (M) and four propionic acid Propionic acid (, from the Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula CH3CH2CO2H. It is a liq ... groups (P). In coproporphyrogen III, the order around the outer ring is MP-MP-MP-PM. For comparison, coproporphyrinogen I has them in the sequence MP-MP-MP-MP. heme. Biosynthesis and metabolism In the main Porphyrin#Synthesis, porphyrin biosynthesis pathway, coproporphyrinogen III is derived f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy groups. Both the negatively charged anion , called hydroxide, and the neutral radical , known as the hydroxyl radical, consist of an unbonded hydroxy group. According to IUPAC definitions, the term ''hydroxyl'' refers to the hydroxyl radical () only, while the functional group is called a ''hydroxy group''. Properties Water, alcohols, carboxylic acids, and many other hydroxy-containing compounds can be readily deprotonated due to a large difference between the electronegativity of oxygen (3.5) and that of hydrogen (2.1). Hydroxy-containing compounds engage in intermolecular hydrogen bonding increasing the electrostatic attraction between molecules and thus to higher boiling and melting points than found for compounds that lack this f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Condensation Reaction
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a dehydration synthesis. However other molecules can also be lost, such as ammonia, ethanol, acetic acid and hydrogen sulfide. The addition of the two molecules typically proceeds in a step-wise fashion to the addition product, usually in equilibrium, and with loss of a water molecule (hence the name condensation). The reaction may otherwise involve the functional groups of the molecule, and is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and to the biosynthesis of fatty acids. Many variations of condensation reactions exist. Common examples include the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |