|
Sirohydrochlorin
Sirohydrochlorin is a tetrapyrrole macrocyclic metabolic intermediate in the biosynthesis of sirohaem, the iron-containing prosthetic group in sulfite reductase enzymes. It is also the biosynthetic precursor to cofactor F430, an enzyme which catalyzes the release of methane in the final step of methanogenesis. Structure Sirohydrochlorin was first isolated in the early 1970s when it was shown to be the metal-free form of the prosthetic group in the ferredoxin-nitrite reductase from spinach. Its chemical identity was established by spectroscopy and by total synthesis. Biosynthesis Sirohydrochlorin is derived from a tetrapyrrolic structural framework created by the enzymes deaminase and cosynthetase which transform aminolevulinic acid via porphobilinogen and hydroxymethylbilane to uroporphyrinogen III. The latter is the first macrocyclic intermediate common to haem, chlorophyll, sirohaem and vitamin B12. Uroporphyrinogen III is subsequently transformed by the addition of two meth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cofactor F430
F430 is the cofactor (sometimes called the coenzyme) of the enzyme methyl coenzyme M reductase (MCR). MCR catalyzes the reaction that releases methane in the final step of methanogenesis: : + HS–CoB → + CoB–S–S–CoM It is found only in methanogenic Archaea and anaerobic methanotrophic Archaea. It occurs in relatively high concentrations in archaea that are involved in reverse methanogenesis: these can contain up to 7% by weight of the nickel protein. Structure The trivial name cofactor F430 was assigned in 1978 based on the properties of a yellow sample extracted from ''Methanobacterium thermoautotrophicum'', which had a spectroscopic maximum at 430 nm. It was identified as the MCR cofactor in 1982 and the complete structure was deduced by X-ray crystallography and NMR spectroscopy. Coenzyme F430 features a reduced porphyrin in a macrocyclic ring system called a corphin. In addition, it possesses two additional rings in comparison to the standard tetrapyrrole ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sirohydrochlorin Cobaltochelatase
The enzyme sirohydrochlorin cobaltochelatase (EC 4.99.1.3) catalyzes the reaction :cobalt-sirohydrochlorin + 2 H+ = sirohydrochlorin + Co2+ In the forward direction of reactions towards cobalamin in anaerobic bacteria, the two substrates of this enzyme are sirohydrochlorin and Co2+; its two products are cobalt-sirohydrochlorin and H+. This enzyme belongs to the family of lyases, specifically the "catch-all" class of lyases that do not fit into any other sub-class. The systematic name of this enzyme class is cobalt-sirohydrochlorin cobalt-lyase (sirohydrochlorin-forming). Other names in common use include CbiK, CbiX, CbiXS, anaerobic cobalt chelatase, cobaltochelatase mbiguous'', and sirohydrochlorin cobalt-lyase (incorrect). This enzyme is part of the biosynthetic pathway to cobalamin (vitamin B12) in bacteria such as ''Salmonella typhimurium'' and ''Bacillus megaterium''. It has also been identified as the enzyme which inserts nickel into sirohydrochlorin in the biosynthes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sirohydrochlorin Ferrochelatase
The enzyme sirohydrochlorin ferrochelatase (EC 4.99.1.4) catalyzes the following reaction: : siroheme + 2H+ \rightleftharpoons sirohydrochlorin + Fe2+ This enzyme belongs to the family of lyases, to be specific the "catch-all" class of lyases that do not fit into any other sub-class. The systematic name of this enzyme class is siroheme ferro-lyase (sirohydrochlorin-forming). The enzyme is also known as SirB and present in all plants and nitrate and sulfate assimilating/dissimilating bacteria. Siroheme is a co-factor of both assimilatory and dissimilatory nitrite and sulfite reductases. Siroheme is synthesized from the central tetrapyrrole molecule uroporphyrinogen III, which forms the first branch-point of tetrapyrrole biosynthetic pathway, the other branch being the heme/chlorophyll branch. The siroheme branch consists of three steps: methylation, dehydrogenation, and ferrochelation, with the last step carried out by sirohydrochlorin ferrochelatase. Sirohydrochlorin ferrochel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrosirohydrochlorin
Dihydrosirohydrochlorin is one of several naturally occurring tetrapyrrole macrocyclic metabolic intermediates in the biosynthesis of vitamin B12 (cobalamin). Its oxidised form, sirohydrochlorin, is precursor to sirohaem, the iron-containing prosthetic group in sulfite reductase enzymes. Further biosynthetic transformations convert sirohydrochlorin to cofactor F430 for an enzyme which catalyzes the release of methane in the final step of methanogenesis. Biosynthesis Dihydrosirohydrochlorin is derived from a tetrapyrrolic structural framework created by the enzymes deaminase and cosynthetase which transform aminolevulinic acid via porphobilinogen and hydroxymethylbilane to uroporphyrinogen III. The latter is the first macrocyclic intermediate common to haem, chlorophyll, sirohaem and vitamin B12. Uroporphyrinogen III is subsequently transformed by the addition of two methyl groups to form dihydrosirohydrochlorin. See also *Cobalamin biosynthesis *Sirohydrochlorin *Precorrin-2 dehy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminolevulinic Acid
δ-Aminolevulinic acid (also dALA, δ-ALA, 5ALA or 5-aminolevulinic acid), an endogenous non-proteinogenic amino acid, is the first compound in the porphyrin synthesis pathway, the pathway that leads to heme in mammals, as well as chlorophyll in plants. 5ALA is used in photodynamic detection and surgery of cancer.Wagnières, G.., Jichlinski, P., Lange, N., Kucera, P., Van den Bergh, H. (2014). Detection of Bladder Cancer by Fluorescence Cystoscopy: From Bench to Bedside - the Hexvix Story. Handbook of Photomedicine, 411-426. Medical uses As a precursor of a photosensitizer, 5ALA is also used as an add-on agent for photodynamic therapy. In contrast to larger photosensitizer molecules, it is predicted by computer simulations to be able to penetrate tumor cell membranes. Cancer diagnosis Photodynamic detection is the use of photosensitive drugs with a light source of the right wavelength for the detection of cancer, using fluorescence of the drug. 5ALA, or derivatives thereof, can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitamin B12
Vitamin B12, also known as cobalamin, is a water-soluble vitamin involved in metabolism. It is one of eight B vitamins. It is required by animals, which use it as a cofactor in DNA synthesis, in both fatty acid and amino acid metabolism. It is important in the normal functioning of the nervous system via its role in the synthesis of myelin, and in the circulatory system in the maturation of red blood cells in the bone marrow. Plants do not need cobalamin and carry out the reactions with enzymes that are not dependent on it. Vitamin B12 is the most chemically complex of all vitamins, and for humans, the only vitamin that must be sourced from animal-derived foods or from supplements. Only some archaea and bacteria can synthesize vitamin B12. Most people in developed countries get enough B12 from the consumption of meat or foods with animal sources. Foods containing vitamin B12 include meat, clams, liver, fish, poultry, eggs, and dairy products. Many breakfast cereals are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to absorb energy from light. Chlorophylls absorb light most strongly in the blue portion of the electromagnetic spectrum as well as the red portion. Conversely, it is a poor absorber of green and near-green portions of the spectrum. Hence chlorophyll-containing tissues appear green because green light, diffusively reflected by structures like cell walls, is less absorbed. Two types of chlorophyll exist in the photosystems of green plants: chlorophyll ''a'' and ''b''. History Chlorophyll was first isolated and named by Joseph Bienaimé Caventou and Pierre Joseph Pelletier in 1817. The presence of magnesium in chlorophyll was discovered in 1906, and was that element's first detection in living tissue. After initial work done by German chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver. In biochemical terms, heme is a coordination complex "consisting of an iron ion coordinated to a porphyrin acting as a tetradentate ligand, and to one or two axial ligands." The definition is loose, and many depictions omit the axial ligands. Among the metalloporphyrins deployed by metalloproteins as prosthetic groups, heme is one of the most widely used and defines a family of proteins known as hemoproteins. Hemes are most commonly recognized as components of hemoglobin, the red pigment in blood, but are also found in a number of other biologically important hemoproteins such as myoglobin, cytochromes, catalases, heme peroxidase, and endothelial nitric oxide synthase. The word ''haem'' is derived from Greek ''haima'' meaning "blood". Function Hemoproteins have diverse biological functions incl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen III
Uroporphyrinogen III is a tetrapyrrole, the first macrocyclic intermediate in the biosynthesis of heme, chlorophyll, vitamin B12, and siroheme. It is a colorless compound, like other porphyrinogens. Structure The molecular structure of uroporphyrinogen III can be described as a hexahydroporphine core, where each pyrrole ring has the hydrogen atoms on its two outermost carbons replaced by an acetic acid group (, "A") and a propionic acid group (, "P"). The groups are attached in an asymmetric way: going around the macrocycle, the order is AP-AP-AP-PA. Biosynthesis and metabolism In the general porphyrin biosynthesis pathway, uroporphyrinogen III is derived from the linear tetrapyrrole preuroporphyrinogen (a substituted hydroxymethylbilane) by the action of the enzyme uroporphyrinogen-III cosynthase. The conversion entails a reversal of the last pyrrole unit (thus swapping the acetic and propionic acid groups) and a condensation reaction that closes the macrocycle by eliminati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxymethylbilane
Hydroxymethylbilane, also known as preuroporphyrinogen, is an organic compound that occurs in living organisms during the synthesis of porphyrins, a group of critical substances that include haemoglobin, myoglobin, and chlorophyll. The name is often abbreviated as HMB. The compound is a substituted bilane, a chain of four pyrrole rings interconnected by methylene bridges . The chain starts with a hydroxymethyl group and ends with an hydrogen, in place of the respective methylene bridges. The other two carbon atoms of each pyrrole cycle are connected to an acetic acid group and a propionic acid group , in that order. The compound is generated from four molecules of porphobilinogen by the enzyme porphobilinogen deaminase: The enzyme uroporphyrinogen III synthase closes the chain to form a porphyrinogen a class of compounds with the hexahydroporphine macrocycle; specifically, uroporphyrinogen III. In the absence of the enzyme, the compound undergoes spontaneous cyclization a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
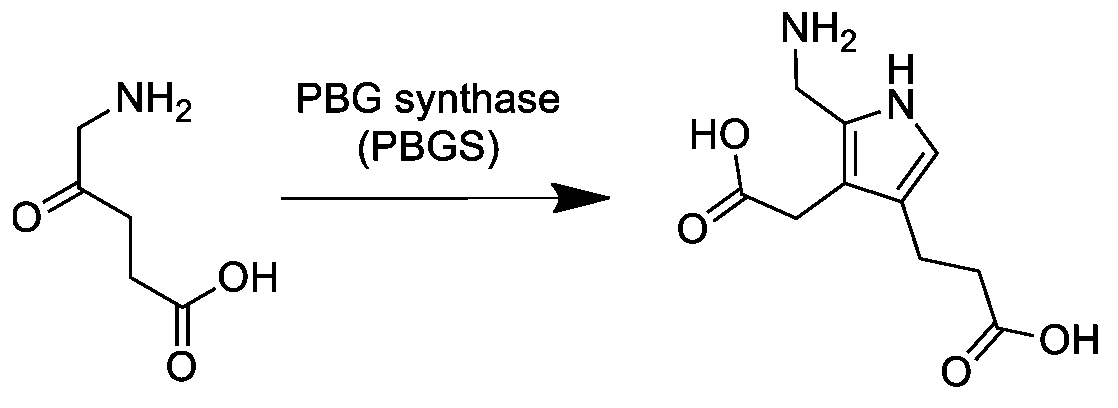
Porphobilinogen
Porphobilinogen (PBG) is an organic compound that occurs in living organisms as an intermediate in the biosynthesis of porphyrins, which include critical substances like hemoglobin and chlorophyll. The structure of the molecule can be described as molecule of pyrrole with sidechains substituted for hydrogen atoms at positions 2, 3 and 4 in the ring (1 being the nitrogen atom); respectively, an aminomethyl group , an acetic acid (carboxymethyl) group , and a propionic acid (carboxyethyl) group . Biosynthesis In the first step of the porphyrin biosynthesis pathway, porphobilinogen is generated from aminolevulinate (ALA) by the enzyme ALA dehydratase. Metabolism In the typical porphyrin biosynthesis pathway, four molecules of porphobilinogen are concatenated by carbons 2 and 5 of the pyrrole ring (adjacent to the nitrogen atom) into hydroxymethyl bilane by the enzyme porphobilinogen deaminase, also known as hydroxymethylbilane synthase. Pathologies Acute intermittent porphyr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrapyrrole
Tetrapyrroles are a class of chemical compounds that contain four pyrrole or pyrrole-like rings. The pyrrole/pyrrole derivatives are linked by ( =- or -- units), in either a linear or a cyclic fashion. Pyrroles are a five-atom ring with four carbon atoms and one nitrogen atom. Tetrapyrroles are common cofactors in biochemistry and their biosynthesis and degradation feature prominently in the chemistry of life. Some tetrapyrroles form the active core of compounds with crucial biochemical roles in living systems, such as hemoglobin and chlorophyll. In these two molecules, in particular, the pyrrole macrocycle ring frames a metal atom, that forms a coordination compound with the pyrroles and plays a central role in the biochemical function of those molecules. Structure Linear tetrapyrroles (called bilanes) include: *Heme breakdown products (e.g., bilirubin, biliverdin) * Phycobilins (found in cyanobacteria) *Luciferins as found in dinoflagellates and euphausiid shrimps (krill) F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |