Chloro on:
[Wikipedia]
[Google]
[Amazon]
Chlorine is a
p. 105:
''"Accipe salis petrae, vitrioli, & alumnis partes aequas: exsiccato singula, & connexis simul, distilla aquam. Quae nil aliud est, quam merum sal volatile. Hujus accipe uncias quatuor, salis armeniaci unciam junge, in forti vitro, alembico, per caementum (ex cera, colophonia, & vitri pulverre) calidissime affusum, firmato; mox, etiam in frigore, Gas excitatur, & vas, utut forte, dissilit cum fragore."'' (Take equal parts of saltpeter .e., sodium nitrate vitriol .e., concentrated sulfuric acid and alum: dry each and combine simultaneously; distill off the water .e., liquid That istillateis nothing else than pure volatile salt .e., spirit of nitre, nitric acid Take four ounces of this iz, nitric acid add one ounce of Armenian salt .e., ammonium chloride lace itin a strong glass alembic sealed by cement ( adefrom wax, rosin, and powdered glass)
p. 408
''"Sal armeniacus enim, & aqua chrysulca, quae singula per se distillari, possunt, & pati calorem: sin autem jungantur, & intepescant, non possunt non, quin statim in Gas sylvestre, sive incoercibilem flatum transmutentur."'' (Truly Armenian salt .e., ammonium chlorideand nitric acid, each of which can be distilled by itself, and submitted to heat; but if, on the other hand, they be combined and become warm, they cannot but be changed immediately into carbon dioxide ote: van Helmont’s identification of the gas is mistakenor an incondensable gas.)
See also:
Helmont, Johannes (Joan) Baptista Van, Encyclopedia.Com
"Others were chlorine gas from the reaction of nitric acid and sal ammoniac; … " * Wisniak, Jaime (2009) "Carl Wilhelm Scheele," ''Revista CENIC Ciencias Químicas'', 40 (3): 165–73 ; see p. 168: "Early in the seventeenth century Johannes Baptiste van Helmont (1579–1644) mentioned that when sal marin (sodium chloride) or sal ammoniacus and aqua chrysulca (nitric acid) were mixed together, a flatus incoercible (non-condensable gas) was evolved."

 Chlorine is the second halogen, being a
Chlorine is the second halogen, being a
 The simplest chlorine compound is
The simplest chlorine compound is
 Nearly all elements in the periodic table form binary chlorides. The exceptions are decidedly in the minority and stem in each case from one of three causes: extreme inertness and reluctance to participate in chemical reactions (the
Nearly all elements in the periodic table form binary chlorides. The exceptions are decidedly in the minority and stem in each case from one of three causes: extreme inertness and reluctance to participate in chemical reactions (the

 The chlorine oxides are well-studied in spite of their instability (all of them are endothermic compounds). They are important because they are produced when
The chlorine oxides are well-studied in spite of their instability (all of them are endothermic compounds). They are important because they are produced when
 Like the other carbon–halogen bonds, the C–Cl bond is a common functional group that forms part of core
Like the other carbon–halogen bonds, the C–Cl bond is a common functional group that forms part of core
 Chlorine is too reactive to occur as the free element in nature but is very abundant in the form of its chloride salts. It is the twenty-first most abundant element in Earth's crust and makes up 126
Chlorine is too reactive to occur as the free element in nature but is very abundant in the form of its chloride salts. It is the twenty-first most abundant element in Earth's crust and makes up 126  In the
In the
The Foul and the Fragrant: Odor and the French Social Imagination
''. Harvard University Press. pp. 121–22. These "putrid miasmas" were thought by many to cause the spread of "contagion" and "infection" – both words used before the germ theory of infection. Chloride of lime was used for destroying odors and "putrid matter". One source claims chloride of lime was used by Dr. John Snow to disinfect water from the cholera-contaminated well that was feeding the Broad Street pump in 1854 London, though three other reputable sources that describe that famous cholera epidemic do not mention the incident.Vinten-Johansen, Peter, Howard Brody, Nigel Paneth, Stephen Rachman and Michael Rip. (2003). ''Cholera, Chloroform, and the Science of Medicine''. New York:Oxford University. One reference makes it clear that chloride of lime was used to disinfect the
 Perhaps the most famous application of Labarraque's chlorine and chemical base solutions was in 1847, when
Perhaps the most famous application of Labarraque's chlorine and chemical base solutions was in 1847, when
 The first continuous application of chlorination to drinking U.S. water was installed in
The first continuous application of chlorination to drinking U.S. water was installed in

Chlorine
at ''The Periodic Table of Videos'' (University of Nottingham) * Agency for Toxic Substances and Disease Registry
Chlorine
Chlorine Production Using Mercury, Environmental Considerations and Alternatives
National Institute for Occupational Safety and Health – Chlorine Page
Chlorine Institute
– Trade association representing the chlorine industry
Chlorine Online
– the web portal of Eurochlor – the business association of the European chlor-alkali industry * {{Authority control Chlorine, Chemical elements Diatomic nonmetals Gases with color Halogens Hazardous air pollutants Industrial gases Occupational safety and health Oxidizing agents Pulmonary agents Reactive nonmetals Swimming pool equipment World Health Organization essential medicines
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Cl and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
17. The second-lightest of the halogens, it appears between fluorine and bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simil ...
in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxid ...
: among the elements, it has the highest electron affinity
The electron affinity (''E''ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.
::X(g) + e− → X−(g) + energy
Note that this is ...
and the third-highest electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
on the revised Pauling scale
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and ...
, behind only oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
and fluorine.
Chlorine played an important role in the experiments conducted by medieval alchemists
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim world, ...
, which commonly involved the heating of chloride salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively ...
like ammonium chloride (sal ammoniac
Salammoniac, also sal ammoniac or salmiac, is a rare naturally occurring mineral composed of ammonium chloride, NH4Cl. It forms colorless, white, or yellow-brown crystals in the isometric-hexoctahedral class. It has very poor cleavage and is ...
) and sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35 ...
(common salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
), producing various chemical substances containing chlorine such as hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride g ...
, mercury(II) chloride (corrosive sublimate), and hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
(in the form of ). However, the nature of free chlorine gas as a separate substance was only recognised around 1630 by Jan Baptist van Helmont
Jan Baptist van Helmont (; ; 12 January 1580 – 30 December 1644) was a chemist, physiologist, and physician from Brussels. He worked during the years just after Paracelsus and the rise of iatrochemistry, and is sometimes considered t ...
. Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hydro ...
wrote a description of chlorine gas in 1774, supposing it to be an oxide of a new element. In 1809, chemists suggested that the gas might be a pure element, and this was confirmed by Sir Humphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for th ...
in 1810, who named it after the Ancient Greek
Ancient Greek includes the forms of the Greek language used in ancient Greece and the ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Dark Ages (), the Archaic p ...
(, "pale green") because of its colour.
Because of its great reactivity, all chlorine in the Earth's crust is in the form of ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
ic chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride s ...
compounds, which includes table salt. It is the second-most abundant halogen (after fluorine) and twenty-first most abundant chemical element in Earth's crust. These crustal deposits are nevertheless dwarfed by the huge reserves of chloride in seawater.
Elemental chlorine is commercially produced from brine
Brine is a high-concentration Solution (chemistry), solution of salt (NaCl) in water (H2O). In diverse contexts, ''brine'' may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of that of ...
by electrolysis, predominantly in the chlor-alkali
The chloralkali process (also chlor-alkali and chlor alkali) is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide (caustic soda), which are commodi ...
process. The high oxidising potential of elemental chlorine led to the development of commercial bleaches and disinfectant
A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than s ...
s, and a reagent for many processes in the chemical industry. Chlorine is used in the manufacture of a wide range of consumer products, about two-thirds of them organic chemicals such as polyvinyl chloride (PVC), many intermediates for the production of plastics
Plastics are a wide range of synthetic or semi-synthetic materials that use polymers as a main ingredient. Their plasticity makes it possible for plastics to be moulded, extruded or pressed into solid objects of various shapes. This adaptab ...
, and other end products which do not contain the element. As a common disinfectant, elemental chlorine and chlorine-generating compounds are used more directly in swimming pool
A swimming pool, swimming bath, wading pool, paddling pool, or simply pool, is a structure designed to hold water to enable Human swimming, swimming or other leisure activities. Pools can be built into the ground (in-ground pools) or built ...
s to keep them sanitary
Sanitation refers to public health conditions related to clean drinking water and treatment and disposal of human excreta and sewage. Preventing human contact with feces is part of sanitation, as is hand washing with soap. Sanitation system ...
. Elemental chlorine at high concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'' ...
is extremely dangerous, and poisonous
Poison is a chemical substance that has a detrimental effect to life. The term is used in a wide range of scientific fields and industries, where it is often specifically defined. It may also be applied colloquially or figuratively, with a broa ...
to most living organisms. As a chemical warfare
Chemical warfare (CW) involves using the toxic properties of chemical substances as weapons. This type of warfare is distinct from nuclear warfare, biological warfare and radiological warfare, which together make up CBRN, the military a ...
agent, chlorine was first used in World War I
World War I (28 July 1914 11 November 1918), often abbreviated as WWI, was one of the deadliest global conflicts in history. Belligerents included much of Europe, the Russian Empire, the United States, and the Ottoman Empire, with fightin ...
as a poison gas
Many gases have toxic properties, which are often assessed using the LC50 (median lethal dose) measure. In the United States, many of these gases have been assigned an NFPA 704 health rating of 4 (may be fatal) or 3 (may cause serious or perm ...
weapon.
In the form of chloride ions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
, chlorine is necessary to all known species of life. Other types of chlorine compounds are rare in living organisms, and artificially produced chlorinated organics range from inert to toxic. In the upper atmosphere Upper atmosphere is a collective term that refers to various layers of the atmosphere of the Earth above the troposphere and corresponding regions of the atmospheres of other planets, and includes:
* The mesosphere, which on Earth lies between the ...
, chlorine-containing organic molecules such as chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and pro ...
s have been implicated in ozone depletion. Small quantities of elemental chlorine are generated by oxidation of chloride ions in neutrophil
Neutrophils (also known as neutrocytes or heterophils) are the most abundant type of granulocytes and make up 40% to 70% of all white blood cells in humans. They form an essential part of the innate immune system, with their functions varying in ...
s as part of an immune system
The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as Tumor immunology, cancer cells and objects such ...
response against bacteria.
History
The most common compound of chlorine, sodium chloride, has been known since ancient times; archaeologists have found evidence thatrock salt
Halite (), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride ( Na Cl). Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, pi ...
was used as early as 3000 BC and brine
Brine is a high-concentration Solution (chemistry), solution of salt (NaCl) in water (H2O). In diverse contexts, ''brine'' may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of that of ...
as early as 6000 BC.
Early discoveries
Around 900, the authors of the Arabic writings attributed toJabir ibn Hayyan
Abū Mūsā Jābir ibn Ḥayyān (Arabic: , variously called al-Ṣūfī, al-Azdī, al-Kūfī, or al-Ṭūsī), died 806−816, is the purported author of an enormous number and variety of works in Arabic, often called the Jabirian corpus. The ...
(Latin: Geber) and the Persian physician and alchemist Abu Bakr al-Razi
Abū Bakr al-Rāzī (full name: ar, أبو بکر محمد بن زکریاء الرازي, translit=Abū Bakr Muḥammad ibn Zakariyyāʾ al-Rāzī, label=none), () rather than ar, زکریاء, label=none (), as for example in , or in . In m ...
( 865–925, Latin: Rhazes) were experimenting with sal ammoniac
Salammoniac, also sal ammoniac or salmiac, is a rare naturally occurring mineral composed of ammonium chloride, NH4Cl. It forms colorless, white, or yellow-brown crystals in the isometric-hexoctahedral class. It has very poor cleavage and is ...
( ammonium chloride), which when it was distilled together with vitriol
Vitriol is the general chemical name encompassing a class of chemical compound comprising sulfates of certain metalsoriginally, iron or copper. Those mineral substances were distinguished by their color, such as green vitriol for hydrated iron(II ...
(hydrated sulfates
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ar ...
of various metals) produced hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride g ...
. However, it appears that in these early experiments with chloride salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively ...
, the gaseous products were discarded, and hydrogen chloride may have been produced many times before it was discovered that it can be put to chemical use. One of the first such uses was the synthesis of mercury(II) chloride (corrosive sublimate), whose production from the heating of mercury either with alum
An alum () is a type of chemical compound, usually a hydrated double sulfate salt of aluminium with the general formula , where is a monovalent cation such as potassium or ammonium. By itself, "alum" often refers to potassium alum, with t ...
and ammonium chloride or with vitriol and sodium chloride was first described in the ''De aluminibus et salibus'' ("On Alums and Salts", an eleventh- or twelfth century Arabic text falsely attributed to Abu Bakr al-Razi and translated into Latin in the second half of the twelfth century by Gerard of Cremona
Gerard of Cremona (Latin: ''Gerardus Cremonensis''; c. 1114 – 1187) was an Italian translator of scientific books from Arabic into Latin. He worked in Toledo, Kingdom of Castile and obtained the Arabic books in the libraries at Toledo. Some of ...
, 1144–1187). Another important development was the discovery by pseudo-Geber
Pseudo-Geber (or "Latin pseudo-Geber") is the presumed author or group of authors responsible for a corpus of pseudepigraphic alchemical writings dating to the late 13th and early 14th centuries. These writings were falsely attributed to Jabir ...
(in the ''De inventione veritatis'', "On the Discovery of Truth", after c. 1300) that by adding ammonium chloride to nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
, a strong solvent capable of dissolving gold (i.e., '' aqua regia'') could be produced. Although ''aqua regia'' is an unstable mixture that continually gives off fumes containing free chlorine gas, this chlorine gas appears to have been ignored until c. 1630, when its nature as a separate gaseous substance was recognised by the Brabantian
Brabantian or Brabantish, also Brabantic or Brabantine ( nl, Brabants, Standard Dutch pronunciation: , ), is a dialect group of the Dutch language. It is named after the historical Duchy of Brabant, which corresponded mainly to the Dutch pro ...
chemist and physician Jan Baptist van Helmont
Jan Baptist van Helmont (; ; 12 January 1580 – 30 December 1644) was a chemist, physiologist, and physician from Brussels. He worked during the years just after Paracelsus and the rise of iatrochemistry, and is sometimes considered t ...
. From ''"Complexionum atque mistionum elementalium figmentum."'' (Formation of combinations and of mixtures of elements), §37p. 105:
''"Accipe salis petrae, vitrioli, & alumnis partes aequas: exsiccato singula, & connexis simul, distilla aquam. Quae nil aliud est, quam merum sal volatile. Hujus accipe uncias quatuor, salis armeniaci unciam junge, in forti vitro, alembico, per caementum (ex cera, colophonia, & vitri pulverre) calidissime affusum, firmato; mox, etiam in frigore, Gas excitatur, & vas, utut forte, dissilit cum fragore."'' (Take equal parts of saltpeter .e., sodium nitrate vitriol .e., concentrated sulfuric acid and alum: dry each and combine simultaneously; distill off the water .e., liquid That istillateis nothing else than pure volatile salt .e., spirit of nitre, nitric acid Take four ounces of this iz, nitric acid add one ounce of Armenian salt .e., ammonium chloride lace itin a strong glass alembic sealed by cement ( adefrom wax, rosin, and powdered glass)
hat has been
A hat is a head covering which is worn for various reasons, including protection against weather conditions, ceremonial reasons such as university graduation, religious reasons, safety, or as a fashion accessory. Hats which incorporate mecha ...
poured very hot; soon, even in the cold, gas is stimulated, and the vessel, however strong, bursts into fragments.) From ''"De Flatibus"'' (On gases)p. 408
''"Sal armeniacus enim, & aqua chrysulca, quae singula per se distillari, possunt, & pati calorem: sin autem jungantur, & intepescant, non possunt non, quin statim in Gas sylvestre, sive incoercibilem flatum transmutentur."'' (Truly Armenian salt .e., ammonium chlorideand nitric acid, each of which can be distilled by itself, and submitted to heat; but if, on the other hand, they be combined and become warm, they cannot but be changed immediately into carbon dioxide ote: van Helmont’s identification of the gas is mistakenor an incondensable gas.)
See also:
Helmont, Johannes (Joan) Baptista Van, Encyclopedia.Com
"Others were chlorine gas from the reaction of nitric acid and sal ammoniac; … " * Wisniak, Jaime (2009) "Carl Wilhelm Scheele," ''Revista CENIC Ciencias Químicas'', 40 (3): 165–73 ; see p. 168: "Early in the seventeenth century Johannes Baptiste van Helmont (1579–1644) mentioned that when sal marin (sodium chloride) or sal ammoniacus and aqua chrysulca (nitric acid) were mixed together, a flatus incoercible (non-condensable gas) was evolved."

Isolation
The element was first studied in detail in 1774 by Swedish chemistCarl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hydro ...
, and he is credited with the discovery. Scheele produced chlorine by reacting MnO2 (as the mineral pyrolusite
Pyrolusite is a mineral consisting essentially of manganese dioxide ( Mn O2) and is important as an ore of manganese.. It is a black, amorphous appearing mineral, often with a granular, fibrous, or columnar structure, sometimes forming reniform c ...
) with HCl:
:4 HCl + MnO2 → MnCl2 + 2 H2O + Cl2
Scheele observed several of the properties of chlorine: the bleaching effect on litmus
Litmus is a water-soluble mixture of different dyes extracted from lichens. It is often absorbed onto filter paper to produce one of the oldest forms of pH indicator, used to test materials for acidity. It is a purple dye that is extract ...
, the deadly effect on insects, the yellow-green color, and the smell similar to aqua regia. He called it "''dephlogisticated muriatic acid air''" since it is a gas (then called "airs") and it came from hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
(then known as "muriatic acid"). He failed to establish chlorine as an element.
Common chemical theory at that time held that an acid is a compound that contains oxygen (remnants of this survive in the German and Dutch names of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
: ''sauerstoff'' or ''zuurstof'', both translating into English as ''acid substance''), so a number of chemists, including Claude Berthollet, suggested that Scheele's ''dephlogisticated muriatic acid air'' must be a combination of oxygen and the yet undiscovered element, ''muriaticum''.
In 1809, Joseph Louis Gay-Lussac
Joseph Louis Gay-Lussac (, , ; 6 December 1778 – 9 May 1850) was a French chemist and physicist. He is known mostly for his discovery that water is made of two parts hydrogen and one part oxygen (with Alexander von Humboldt), for two laws ...
and Louis-Jacques Thénard tried to decompose ''dephlogisticated muriatic acid air'' by reacting it with charcoal to release the free element ''muriaticum'' (and carbon dioxide). They did not succeed and published a report in which they considered the possibility that ''dephlogisticated muriatic acid air'' is an element, but were not convinced.
In 1810, Sir Humphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for th ...
tried the same experiment again, and concluded that the substance was an element, and not a compound. He announced his results to the Royal Society on 15 November that year. At that time, he named this new element "chlorine", from the Greek word χλωρος (''chlōros'', "green-yellow"), in reference to its color. The name " halogen", meaning "salt producer", was originally used for chlorine in 1811 by Johann Salomo Christoph Schweigger. This term was later used as a generic term to describe all the elements in the chlorine family (fluorine, bromine, iodine), after a suggestion by Jöns Jakob Berzelius
Jöns is a Swedish given name and a surname.
Notable people with the given name include:
* Jöns Jacob Berzelius
Baron Jöns Jacob Berzelius (; by himself and his contemporaries named only Jacob Berzelius, 20 August 1779 – 7 August 1848) ...
in 1826. In 1823, Michael Faraday
Michael Faraday (; 22 September 1791 – 25 August 1867) was an English scientist who contributed to the study of electromagnetism and electrochemistry. His main discoveries include the principles underlying electromagnetic inductio ...
liquefied chlorine for the first time, and demonstrated that what was then known as "solid chlorine" had a structure of chlorine hydrate (Cl2·H2O).
Later uses
Chlorine gas was first used by French chemist Claude Berthollet to bleach textiles in 1785. Modern bleaches resulted from further work by Berthollet, who first producedsodium hypochlorite
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an inorganic chemical compound with the formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may also be viewed as the sodium salt of ...
in 1789 in his laboratory in the town of Javel (now part of Paris
Paris () is the capital and most populous city of France, with an estimated population of 2,165,423 residents in 2019 in an area of more than 105 km² (41 sq mi), making it the 30th most densely populated city in the world in 2020. ...
, France), by passing chlorine gas through a solution of sodium carbonate. The resulting liquid, known as "''Eau de Javel''" ("Javel water
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color (whitening) from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically, to ...
"), was a weak solution of sodium hypochlorite
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an inorganic chemical compound with the formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may also be viewed as the sodium salt of ...
. This process was not very efficient, and alternative production methods were sought. Scottish chemist and industrialist Charles Tennant
Charles Tennant (3 May 1768 – 1 October 1838) was a Scottish chemist and industrialist. He discovered bleaching powder and founded an industrial dynasty.
Biography
Charles Tennant was born at Laigh Corton, Alloway, Ayrshire, the sixth of t ...
first produced a solution of calcium hypochlorite
Calcium hypochlorite is an inorganic compound with formula Ca(OCl)2. It is the main active ingredient of commercial products called bleaching powder, chlorine powder, or chlorinated lime, used for water treatment and as a bleaching agent. Th ...
("chlorinated lime"), then solid calcium hypochlorite (bleaching powder). These compounds produced low levels of elemental chlorine and could be more efficiently transported than sodium hypochlorite, which remained as dilute solutions because when purified to eliminate water, it became a dangerously powerful and unstable oxidizer. Near the end of the nineteenth century, E. S. Smith patented a method of sodium hypochlorite production involving electrolysis of brine
Brine is a high-concentration Solution (chemistry), solution of salt (NaCl) in water (H2O). In diverse contexts, ''brine'' may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of that of ...
to produce sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkal ...
and chlorine gas, which then mixed to form sodium hypochlorite. This is known as the chloralkali process
The chloralkali process (also chlor-alkali and chlor alkali) is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide (caustic soda), which are commodit ...
, first introduced on an industrial scale in 1892, and now the source of most elemental chlorine and sodium hydroxide. In 1884 Chemischen Fabrik Griesheim of Germany developed another chloralkali process
The chloralkali process (also chlor-alkali and chlor alkali) is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide (caustic soda), which are commodit ...
which entered commercial production in 1888.
Elemental chlorine solutions dissolved in chemically basic water (sodium and calcium hypochlorite
Calcium hypochlorite is an inorganic compound with formula Ca(OCl)2. It is the main active ingredient of commercial products called bleaching powder, chlorine powder, or chlorinated lime, used for water treatment and as a bleaching agent. Th ...
) were first used as anti-putrefaction
Putrefaction is the fifth stage of death, following pallor mortis, algor mortis, rigor mortis, and livor mortis. This process references the breaking down of a body of an animal, such as a human, post-mortem. In broad terms, it can be vie ...
agents and disinfectant
A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than s ...
s in the 1820s, in France, long before the establishment of the germ theory of disease
The germ theory of disease is the currently accepted scientific theory for many diseases. It states that microorganisms known as pathogens or "germs" can lead to disease. These small organisms, too small to be seen without magnification, invade ...
. This practice was pioneered by Antoine-Germain Labarraque, who adapted Berthollet's "Javel water" bleach and other chlorine preparations (for a more complete history, see below). Elemental chlorine has since served a continuous function in topical antisepsis
An antiseptic (from Greek ἀντί ''anti'', "against" and σηπτικός ''sēptikos'', "putrefactive") is an antimicrobial substance or compound that is applied to living tissue/skin to reduce the possibility of infection, sepsis, or putre ...
(wound irrigation solutions and the like) and public sanitation, particularly in swimming and drinking water.
Chlorine gas was first used as a weapon on April 22, 1915, at Ypres
Ypres ( , ; nl, Ieper ; vls, Yper; german: Ypern ) is a Belgian city and municipality in the province of West Flanders. Though
the Dutch name is the official one, the city's French name is most commonly used in English. The municipality ...
by the German Army. The effect on the allies was devastating because the existing gas mask
A gas mask is a mask used to protect the wearer from inhaling airborne pollutants and toxic gases. The mask forms a sealed cover over the nose and mouth, but may also cover the eyes and other vulnerable soft tissues of the face. Most gas mas ...
s were difficult to deploy and had not been broadly distributed.
Properties

 Chlorine is the second halogen, being a
Chlorine is the second halogen, being a nonmetal
In chemistry, a nonmetal is a chemical element that generally lacks a predominance of metallic properties; they range from colorless gases (like hydrogen) to shiny solids (like carbon, as graphite). The electrons in nonmetals behave differen ...
in group 17 of the periodic table. Its properties are thus similar to fluorine, bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simil ...
, and iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
, and are largely intermediate between those of the first two. Chlorine has the electron configuration es23p5, with the seven electrons in the third and outermost shell acting as its valence electron
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair f ...
s. Like all halogens, it is thus one electron short of a full octet, and is hence a strong oxidising agent, reacting with many elements in order to complete its outer shell. Corresponding to periodic trend
Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. They were discovered by the Russian chemist Dmitri Mendeleev in the year 1863. Major periodic trends include atom ...
s, it is intermediate in electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
between fluorine and bromine (F: 3.98, Cl: 3.16, Br: 2.96, I: 2.66), and is less reactive than fluorine and more reactive than bromine. It is also a weaker oxidising agent than fluorine, but a stronger one than bromine. Conversely, the chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride s ...
ion is a weaker reducing agent than bromide, but a stronger one than fluoride. It is intermediate in atomic radius
The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Since the boundary is not a well-defined physical entity, th ...
between fluorine and bromine, and this leads to many of its atomic properties similarly continuing the trend from iodine to bromine upward, such as first ionisation energy
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule ...
, electron affinity
The electron affinity (''E''ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.
::X(g) + e− → X−(g) + energy
Note that this is ...
, enthalpy of dissociation of the X2 molecule (X = Cl, Br, I), ionic radius, and X–X bond length. (Fluorine is anomalous due to its small size.)
All four stable halogens experience intermolecular van der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and t ...
s of attraction, and their strength increases together with the number of electrons among all homonuclear diatomic halogen molecules. Thus, the melting and boiling points of chlorine are intermediate between those of fluorine and bromine: chlorine melts at −101.0 °C and boils at −34.0 °C. As a result of the increasing molecular weight of the halogens down the group, the density and heats of fusion and vaporisation of chlorine are again intermediate between those of bromine and fluorine, although all their heats of vaporisation are fairly low (leading to high volatility) thanks to their diatomic molecular structure. The halogens darken in colour as the group is descended: thus, while fluorine is a pale yellow gas, chlorine is distinctly yellow-green. This trend occurs because the wavelengths of visible light absorbed by the halogens increase down the group. Specifically, the colour of a halogen, such as chlorine, results from the electron transition between the highest occupied antibonding ''πg'' molecular orbital and the lowest vacant antibonding ''σu'' molecular orbital. The colour fades at low temperatures, so that solid chlorine at −195 °C is almost colourless.
Like solid bromine and iodine, solid chlorine crystallises in the orthorhombic crystal system
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a ...
, in a layered lattice of Cl2 molecules. The Cl–Cl distance is 198 pm (close to the gaseous Cl–Cl distance of 199 pm) and the Cl···Cl distance between molecules is 332 pm within a layer and 382 pm between layers (compare the van der Waals radius of chlorine, 180 pm). This structure means that chlorine is a very poor conductor of electricity, and indeed its conductivity is so low as to be practically unmeasurable.
Isotopes
Chlorine has two stable isotopes, 35Cl and 37Cl. These are its only two natural isotopes occurring in quantity, with 35Cl making up 76% of natural chlorine and 37Cl making up the remaining 24%. Both are synthesised in stars in the oxygen-burning andsilicon-burning process
In astrophysics, silicon burning is a very brief sequence of nuclear fusion reactions that occur in massive stars with a minimum of about 8–11 solar masses. Silicon burning is the final stage of fusion for massive stars that have run out of the f ...
es. Both have nuclear spin 3/2+ and thus may be used for nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
, although the spin magnitude being greater than 1/2 results in non-spherical nuclear charge distribution and thus resonance broadening as a result of a nonzero nuclear quadrupole moment Nuclear quadrupole resonance spectroscopy or NQR is a chemical analysis technique related to nuclear magnetic resonance ( NMR). Unlike NMR, NQR transitions of nuclei can be detected in the absence of a magnetic field, and for this reason NQR spectr ...
and resultant quadrupolar relaxation. The other chlorine isotopes are all radioactive, with half-lives
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ato ...
too short to occur in nature primordially. Of these, the most commonly used in the laboratory are 36Cl (''t''1/2 = 3.0×105 y) and 38Cl (''t''1/2 = 37.2 min), which may be produced from the neutron activation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emitti ...
of natural chlorine.
The most stable chlorine radioisotope is 36Cl. The primary decay mode of isotopes lighter than 35Cl is electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. T ...
to isotopes of sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
; that of isotopes heavier than 37Cl is beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For e ...
to isotopes of argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as a ...
; and 36Cl may decay by either mode to stable 36S or 36Ar. 36Cl occurs in trace quantities in nature as a cosmogenic nuclide
Cosmogenic nuclides (or cosmogenic isotopes) are rare nuclides ( isotopes) created when a high-energy cosmic ray interacts with the nucleus of an '' in situ'' Solar System atom, causing nucleons (protons and neutrons) to be expelled from the at ...
in a ratio of about (7–10) × 10−13 to 1 with stable chlorine isotopes: it is produced in the atmosphere by spallation
Spallation is a process in which fragments of material ( spall) are ejected from a body due to impact or stress. In the context of impact mechanics it describes ejection of material from a target during impact by a projectile. In planetary p ...
of 36 Ar by interactions with cosmic ray
Cosmic rays are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the Solar System in our ow ...
protons. In the top meter of the lithosphere, 36Cl is generated primarily by thermal neutron
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium with ...
activation of 35Cl and spallation of 39 K and 40 Ca. In the subsurface environment, muon capture by 40 Ca becomes more important as a way to generate 36Cl.
Chemistry and compounds
Chlorine is intermediate in reactivity between fluorine and bromine, and is one of the most reactive elements. Chlorine is a weaker oxidising agent than fluorine but a stronger one than bromine or iodine. This can be seen from thestandard electrode potential
In electrochemistry, standard electrode potential E^\ominus, or E^\ominus_, is a measure of the reducing power of any element or compound. The IUPAC "Gold Book" defines it as: ''"the value of the standard emf (electromotive force) of a cell in wh ...
s of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). However, this trend is not shown in the bond energies because fluorine is singular due to its small size, low polarisability, and inability to show hypervalence. As another difference, chlorine has a significant chemistry in positive oxidation states while fluorine does not. Chlorination often leads to higher oxidation states than bromination or iodination but lower oxidation states than fluorination. Chlorine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Cl bonds.
Given that E°(O2/H2O) = +1.229 V, which is less than +1.395 V, it would be expected that chlorine should be able to oxidise water to oxygen and hydrochloric acid. However, the kinetics of this reaction are unfavorable, and there is also a bubble overpotential
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly relat ...
effect to consider, so that electrolysis of aqueous chloride solutions evolves chlorine gas and not oxygen gas, a fact that is very useful for the industrial production of chlorine.
Hydrogen chloride
 The simplest chlorine compound is
The simplest chlorine compound is hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride g ...
, HCl, a major chemical in industry as well as in the laboratory, both as a gas and dissolved in water as hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
. It is often produced by burning hydrogen gas in chlorine gas, or as a byproduct of chlorinating hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s. Another approach is to treat sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35 ...
with concentrated sulfuric acid to produce hydrochloric acid, also known as the "salt-cake" process:
:NaCl + H2SO4 NaHSO4 + HCl
:NaCl + NaHSO4 Na2SO4 + HCl
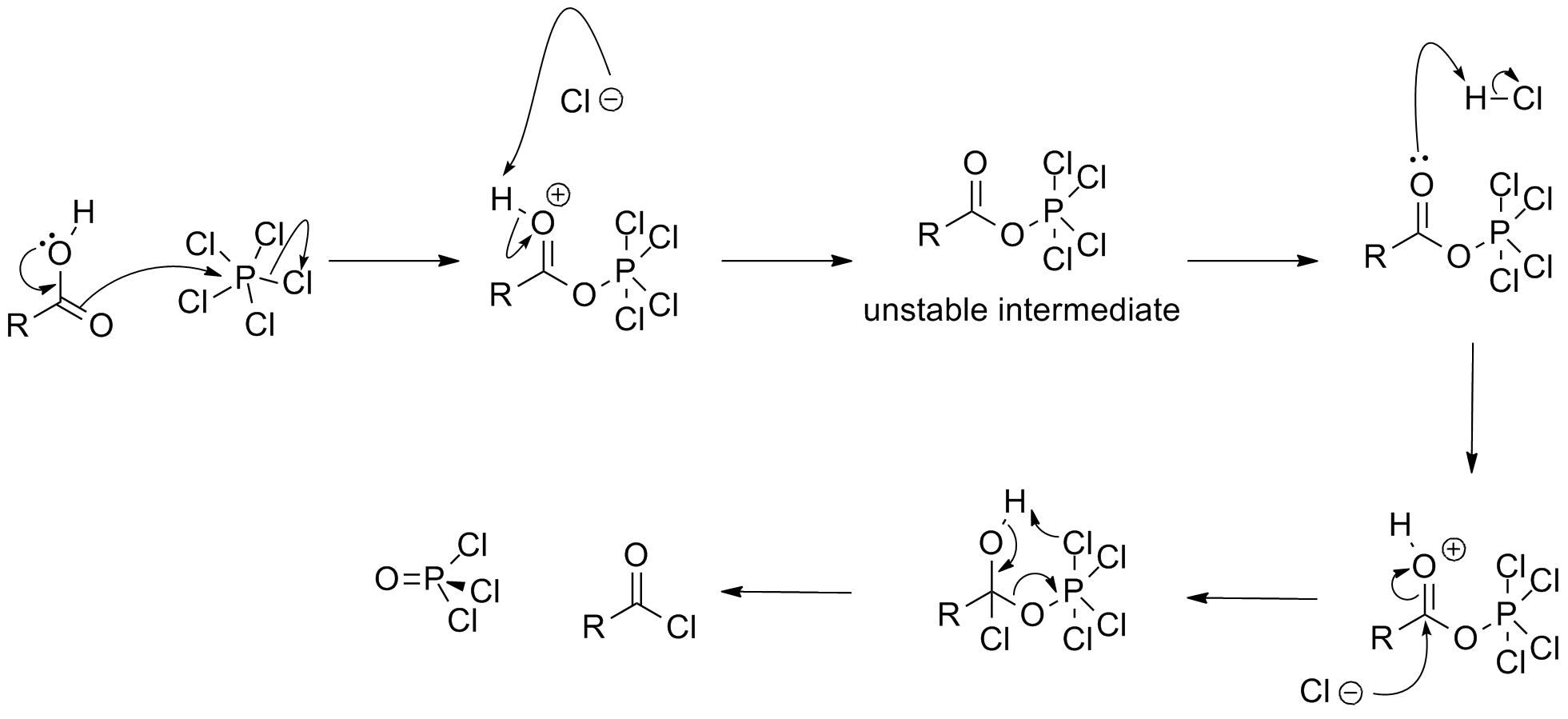
In the laboratory, hydrogen chloride gas may be made by drying the acid with concentrated sulfuric acid. Deuterium chloride, DCl, may be produced by reacting benzoyl chloride
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula . It is a colourless, fuming liquid with an irritating odour, and consists of a benzene ring () with an acyl chloride () substituent. It i ...
with heavy water (D2O).
At room temperature, hydrogen chloride is a colourless gas, like all the hydrogen halides apart from hydrogen fluoride, since hydrogen cannot form strong hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing ...
s to the larger electronegative chlorine atom; however, weak hydrogen bonding is present in solid crystalline hydrogen chloride at low temperatures, similar to the hydrogen fluoride structure, before disorder begins to prevail as the temperature is raised. Hydrochloric acid is a strong acid (p''K''a = −7) because the hydrogen bonds to chlorine are too weak to inhibit dissociation. The HCl/H2O system has many hydrates HCl·''n''H2O for ''n'' = 1, 2, 3, 4, and 6. Beyond a 1:1 mixture of HCl and H2O, the system separates completely into two separate liquid phases. Hydrochloric acid forms an azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This ...
with boiling point 108.58 °C at 20.22 g HCl per 100 g solution; thus hydrochloric acid cannot be concentrated beyond this point by distillation.
Unlike hydrogen fluoride, anhydrous liquid hydrogen chloride is difficult to work with as a solvent, because its boiling point is low, it has a small liquid range, its dielectric constant
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric constant of an insulat ...
is low and it does not dissociate appreciably into H2Cl+ and ions – the latter, in any case, are much less stable than the bifluoride
The bifluoride ion is an inorganic anion with the chemical formula . The anion is colorless. Salts of bifluoride are commonly encountered in the reactions of fluoride salts with hydrofluoric acid. The commercial production of fluorine involves ...
ions () due to the very weak hydrogen bonding between hydrogen and chlorine, though its salts with very large and weakly polarising cations such as Cs+ and (R = Me, Et, Bu''n'') may still be isolated. Anhydrous hydrogen chloride is a poor solvent, only able to dissolve small molecular compounds such as nitrosyl chloride
Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is commonly encountered as a component of aqua regia, a mixture of 3 parts concentrated hydrochloric acid and 1 part of concentrated nitric acid. It is a st ...
and phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it ...
, or salts with very low lattice energies
In chemistry, the lattice energy is the energy change upon formation of one mole of a crystalline ionic compound from its constituent ions, which are assumed to initially be in the gaseous state. It is a measure of the cohesive forces that bi ...
such as tetraalkylammonium halides. It readily protonates electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that ca ...
s containing lone-pairs or π bonds. Solvolysis
In chemistry, solvolysis is a type of nucleophilic substitution (S1/S2) or elimination where the nucleophile is a solvent molecule. Characteristic of S1 reactions, solvolysis of a chiral reactant affords the racemate. Sometimes however, the ster ...
, ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
replacement reactions, and oxidations are well-characterised in hydrogen chloride solution:
:Ph3SnCl + HCl ⟶ Ph2SnCl2 + PhH (solvolysis)
:Ph3COH + 3 HCl ⟶ + H3O+Cl− (solvolysis)
: + BCl3 ⟶ + HCl (ligand replacement)
:PCl3 + Cl2 + HCl ⟶ (oxidation)
Other binary chlorides
 Nearly all elements in the periodic table form binary chlorides. The exceptions are decidedly in the minority and stem in each case from one of three causes: extreme inertness and reluctance to participate in chemical reactions (the
Nearly all elements in the periodic table form binary chlorides. The exceptions are decidedly in the minority and stem in each case from one of three causes: extreme inertness and reluctance to participate in chemical reactions (the noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low che ...
es, with the exception of xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
in the highly unstable XeCl2 and XeCl4); extreme nuclear instability hampering chemical investigation before decay and transmutation (many of the heaviest elements beyond bismuth
Bismuth is a chemical element with the symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs ...
); and having an electronegativity higher than chlorine's (oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
and fluorine) so that the resultant binary compounds are formally not chlorides but rather oxides or fluorides of chlorine. Even though nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seve ...
in NCl3 is bearing a negative charge, the compound is usually called nitrogen trichloride
Nitrogen trichloride, also known as trichloramine, is the chemical compound with the formula NCl3. This yellow, oily, pungent-smelling and explosive liquid is most commonly encountered as a byproduct of chemical reactions between ammonia-derivativ ...
.
Chlorination of metals with Cl2 usually leads to a higher oxidation state than bromination with Br2 when multiple oxidation states are available, such as in MoCl5 and MoBr3. Chlorides can be made by reaction of an element or its oxide, hydroxide, or carbonate with hydrochloric acid, and then dehydrated by mildly high temperatures combined with either low pressure or anhydrous hydrogen chloride gas. These methods work best when the chloride product is stable to hydrolysis; otherwise, the possibilities include high-temperature oxidative chlorination of the element with chlorine or hydrogen chloride, high-temperature chlorination of a metal oxide or other halide by chlorine, a volatile metal chloride, carbon tetrachloride
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemi ...
, or an organic chloride. For instance, zirconium dioxide
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant s ...
reacts with chlorine at standard conditions to produce zirconium tetrachloride
Zirconium(IV) chloride, also known as zirconium tetrachloride, () is an inorganic compound frequently used as a precursor to other compounds of zirconium. This white high-melting solid hydrolyzes rapidly in humid air.
Structure
Unlike molecular T ...
, and uranium trioxide
Uranium trioxide (UO3), also called uranyl oxide, uranium(VI) oxide, and uranic oxide, is the hexavalent oxide of uranium. The solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph, γ-UO3, is a ...
reacts with hexachloropropene when heated under reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reaction ...
to give uranium tetrachloride
Uranium tetrachloride is an inorganic compound, a salt of uranium and chlorine, with the formula UCl4. It is a hygroscopic olive-green solid. It was used in the electromagnetic isotope separation (EMIS) process of uranium enrichment. It is one o ...
. The second example also involves a reduction in oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. ...
, which can also be achieved by reducing a higher chloride using hydrogen or a metal as a reducing agent. This may also be achieved by thermal decomposition or disproportionation as follows:
: EuCl3 + H2 ⟶ EuCl2 + HCl
: ReCl5 ReCl3 + Cl2
: AuCl3 AuCl + Cl2
Most metal chlorides with the metal in low oxidation states (+1 to +3) are ionic. Nonmetals tend to form covalent molecular chlorides, as do metals in high oxidation states from +3 and above. Both ionic and covalent chlorides are known for metals in oxidation state +3 (e.g. scandium chloride is mostly ionic, but aluminium chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms hexahydrate with the formula , containing six water molecules of hydration. Both are colourless crystals, but samples are often contam ...
is not). Silver chloride
Silver chloride is a chemical compound with the chemical formula Ag Cl. This white crystalline solid is well known for its low solubility in water (this behavior being reminiscent of the chlorides of Tl+ and Pb2+). Upon illumination or heating, ...
is very insoluble in water and is thus often used as a qualitative test for chlorine.
Polychlorine compounds
Although dichlorine is a strong oxidising agent with a high first ionisation energy, it may be oxidised under extreme conditions to form the cation. This is very unstable and has only been characterised by its electronic band spectrum when produced in a low-pressure discharge tube. The yellow cation is more stable and may be produced as follows: :Cl2 + ClF + AsF5 This reaction is conducted in the oxidising solventarsenic pentafluoride
Arsenic pentafluoride is a chemical compound of arsenic and fluorine. It is a toxic, colorless gas. The oxidation state of arsenic is +5.
Synthesis
Arsenic pentafluoride can be prepared by direct combination of arsenic and fluorine:
:2As + 5F2 ...
. The trichloride anion, , has also been characterised; it is analogous to triiodide
In chemistry, triiodide usually refers to the triiodide ion, . This anion, one of the polyhalogen ions, is composed of three iodine atoms. It is formed by combining aqueous solutions of iodide salts and iodine. Some salts of the anion have been ...
.
Chlorine fluorides
The three fluorides of chlorine form a subset of theinterhalogen
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms ( fluorine, chlorine, bromine, iodine, or astatine) and no atoms of elements from any other group.
Most interhalogen compounds known are bi ...
compounds, all of which are diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted ...
. Some cationic and anionic derivatives are known, such as , , , and Cl2F+. Some pseudohalides of chlorine are also known, such as cyanogen chloride
Cyanogen chloride is a highly toxic chemical compound with the formula CNCl. This linear, triatomic pseudohalogen is an easily condensed colorless gas. More commonly encountered in the laboratory is the related compound cyanogen bromide, a room-t ...
(ClCN, linear), chlorine cyanate
Cyanate is an anion with the structural formula , usually written . It also refers to any salt containing it, such as ammonium cyanate.
It is an isomer of the much less stable fulminate anion .William R. Martin and David W. Ball (2019): "Sm ...
(ClNCO), chlorine thiocyanate
Thiocyanate (also known as rhodanide) is the anion . It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) thiocyanate was formerly used in pyro ...
(ClSCN, unlike its oxygen counterpart), and chlorine azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant ...
(ClN3).
Chlorine monofluoride (ClF) is extremely thermally stable, and is sold commercially in 500-gram steel lecture bottles. It is a colourless gas that melts at −155.6 °C and boils at −100.1 °C. It may be produced by the direction of its elements at 225 °C, though it must then be separated and purified from chlorine trifluoride
Chlorine trifluoride is an interhalogen compound with the formula ClF3. This colorless, poisonous, corrosive, and extremely reactive gas condenses to a pale-greenish yellow liquid, the form in which it is most often sold (pressurized at room te ...
and its reactants. Its properties are mostly intermediate between those of chlorine and fluorine. It will react with many metals and nonmetals from room temperature and above, fluorinating them and liberating chlorine. It will also act as a chlorofluorinating agent, adding chlorine and fluorine across a multiple bond or by oxidation: for example, it will attack carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
to form carbonyl chlorofluoride, COFCl. It will react analogously with hexafluoroacetone
Hexafluoroacetone (HFA) is a chemical compound with the formula (CF3)2CO. It is structurally similar to acetone; however, its reactivity is markedly different. It a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a mus ...
, (CF3)2CO, with a potassium fluoride
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali halide and occurs naturally as the rare ...
catalyst to produce heptafluoroisopropyl hypochlorite, (CF3)2CFOCl; with nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix '' cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including me ...
s RCN to produce RCF2NCl2; and with the sulfur oxides SO2 and SO3 to produce ClSO2F and ClOSO2F respectively. It will also react exothermically with compounds containing –OH and –NH groups, such as water:
:H2O + 2 ClF ⟶ 2 HF + Cl2O
Chlorine trifluoride
Chlorine trifluoride is an interhalogen compound with the formula ClF3. This colorless, poisonous, corrosive, and extremely reactive gas condenses to a pale-greenish yellow liquid, the form in which it is most often sold (pressurized at room te ...
(ClF3) is a volatile colourless molecular liquid which melts at −76.3 °C and boils at 11.8 °C. It may be formed by directly fluorinating gaseous chlorine or chlorine monofluoride at 200–300 °C. One of the most reactive chemical compounds known, the list of elements it sets on fire is diverse, containing hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
, potassium
Potassium is the chemical element with the symbol K (from Neo-Latin '' kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosp ...
, phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ea ...
, arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, bu ...
, antimony
Antimony is a chemical element with the symbol Sb (from la, stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient ti ...
, sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
, selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and telluriu ...
, tellurium
Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally fo ...
, bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simil ...
, iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
, and powdered molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with le ...
, tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isol ...
, rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring ...
, iridium
Iridium is a chemical element with the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal (after osmium) with a density of ...
, and iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
. It will also ignite water, along with many substances which in ordinary circumstances would be considered chemically inert such as asbestos, concrete, glass, and sand. When heated, it will even corrode noble metal
A noble metal is ordinarily regarded as a metallic chemical element that is generally resistant to corrosion and is usually found in nature in its raw form. Gold, platinum, and the other platinum group metals (ruthenium, rhodium, palladium, ...
s as palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself nam ...
, platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Pla ...
, and gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile ...
, and even the noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low che ...
es xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
and radon
Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colourless, odourless, tasteless noble gas. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chains through ...
do not escape fluorination. An impermeable fluoride layer is formed by sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
, magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
, aluminium
Aluminium (aluminum in AmE, American and CanE, Canadian English) is a chemical element with the Symbol (chemistry), symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately o ...
, zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic t ...
, tin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, ...
, and silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
, which may be removed by heating. Nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
, copper, and steel containers are usually used due to their great resistance to attack by chlorine trifluoride, stemming from the formation of an unreactive layer of metal fluoride. Its reaction with hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazin ...
to form hydrogen fluoride, nitrogen, and chlorine gases was used in experimental rocket engine, but has problems largely stemming from its extreme hypergolicity resulting in ignition without any measurable delay. Today, it is mostly used in nuclear fuel processing, to oxidise uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly ...
to uranium hexafluoride
Uranium hexafluoride (), (sometimes called "hex") is an inorganic compound with the formula UF6. Uranium hexafluoride is a volatile white solid that reacts with water, releasing corrosive hydrofluoric acid. The compound reacts mildly with alumin ...
for its enriching and to separate it from plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhib ...
, as well as in the semiconductor industry, where it is used to clean chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (subst ...
chambers. It can act as a fluoride ion donor or acceptor (Lewis base or acid), although it does not dissociate appreciably into and ions.
Chlorine pentafluoride
Chlorine pentafluoride is an interhalogen compound with formula ClF5. This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry, as confirmed by ...
(ClF5) is made on a large scale by direct fluorination of chlorine with excess fluorine gas at 350 °C and 250 atm, and on a small scale by reacting metal chlorides with fluorine gas at 100–300 °C. It melts at −103 °C and boils at −13.1 °C. It is a very strong fluorinating agent, although it is still not as effective as chlorine trifluoride. Only a few specific stoichiometric reactions have been characterised. Arsenic pentafluoride
Arsenic pentafluoride is a chemical compound of arsenic and fluorine. It is a toxic, colorless gas. The oxidation state of arsenic is +5.
Synthesis
Arsenic pentafluoride can be prepared by direct combination of arsenic and fluorine:
:2As + 5F2 ...
and antimony pentafluoride form ionic adducts of the form lF4sup>+ F6sup>− (M = As, Sb) and water reacts vigorously as follows:
:2 H2O + ClF5 ⟶ 4 HF + FClO2
The product, chloryl fluoride
Chloryl fluoride is the chemical compound with the formula ClO2F. It is commonly encountered as side-product in reactions of chlorine fluorides with oxygen sources. It is the acyl fluoride
In organic chemistry, an acyl halide (also known as an ...
, is one of the five known chlorine oxide fluorides. These range from the thermally unstable FClO to the chemically unreactive perchloryl fluoride (FClO3), the other three being FClO2, F3ClO, and F3ClO2. All five behave similarly to the chlorine fluorides, both structurally and chemically, and may act as Lewis acids or bases by gaining or losing fluoride ions respectively or as very strong oxidising and fluorinating agents.
Chlorine oxides

 The chlorine oxides are well-studied in spite of their instability (all of them are endothermic compounds). They are important because they are produced when
The chlorine oxides are well-studied in spite of their instability (all of them are endothermic compounds). They are important because they are produced when chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and pro ...
s undergo photolysis in the upper atmosphere and cause the destruction of the ozone layer. None of them can be made from directly reacting the elements.
Dichlorine monoxide
Dichlorine monoxide is an inorganic compound with the molecular formula Cl2O. It was first synthesised in 1834 by Antoine Jérôme Balard, who along with Gay-Lussac also determined its composition. In older literature it is often referred to as c ...
(Cl2O) is a brownish-yellow gas (red-brown when solid or liquid) which may be obtained by reacting chlorine gas with yellow mercury(II) oxide
Mercury(II) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula Hg O. It has a red or orange color. Mercury(II) oxide is a solid at room temperature and pressure. The mineral form montroydite is ...
. It is very soluble in water, in which it is in equilibrium with hypochlorous acid
Hypochlorous acid (HClO, HOCl, or ClHO) is a weak acid that forms when chlorine dissolves in water, and itself partially dissociates, forming hypochlorite, ClO−. HClO and ClO− are oxidizers, and the primary disinfection agents of chlorine so ...
(HOCl), of which it is the anhydride. It is thus an effective bleach and is mostly used to make hypochlorite
In chemistry, hypochlorite is an anion with the chemical formula ClO−. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite (household bleach) and calcium hypochlorite (a component of ble ...
s. It explodes on heating or sparking or in the presence of ammonia gas.
Chlorine dioxide
Chlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between 11 °C and −59 °C, and as bright orange crystals below −59 °C. It is usually ...
(ClO2) was the first chlorine oxide to be discovered in 1811 by Humphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for the ...
. It is a yellow paramagnetic gas (deep-red as a solid or liquid), as expected from its having an odd number of electrons: it is stable towards dimerisation due to the delocalisation of the unpaired electron. It explodes above −40 °C as a liquid and under pressure as a gas and therefore must be made at low concentrations for wood-pulp bleaching and water treatment. It is usually prepared by reducing a chlorate
The chlorate anion has the formula ClO3-. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followe ...
as follows:
: + Cl− + 2 H+ ⟶ ClO2 + Cl2 + H2O
Its production is thus intimately linked to the redox reactions of the chlorine oxoacids. It is a strong oxidising agent, reacting with sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
, phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ea ...
, phosphorus halides, and potassium borohydride
Borohydride refers to the anion , which is also called tetrahydroborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing , where ''n'' is an integer from 0 to 3, for example cyanoborohydride or cyanotrihydr ...
. It dissolves exothermically in water to form dark-green solutions that very slowly decompose in the dark. Crystalline clathrate hydrates ClO2·''n''H2O (''n'' ≈ 6–10) separate out at low temperatures. However, in the presence of light, these solutions rapidly photodecompose to form a mixture of chloric and hydrochloric acids. Photolysis of individual ClO2 molecules result in the radicals ClO and ClOO, while at room temperature mostly chlorine, oxygen, and some ClO3 and Cl2O6 are produced. Cl2O3 is also produced when photolysing the solid at −78 °C: it is a dark brown solid that explodes below 0 °C. The ClO radical leads to the depletion of atmospheric ozone and is thus environmentally important as follows:
:Cl• + O3 ⟶ ClO• + O2
:ClO• + O• ⟶ Cl• + O2
Chlorine perchlorate
Chlorine perchlorate is a chemical compound with the formula Cl2O4. This chlorine oxide is an asymmetric oxide, with one chlorine atom in +1 oxidation state and the other +7, with proper formula ClOClO3. It is produced by the photodimerizatio ...
(ClOClO3) is a pale yellow liquid that is less stable than ClO2 and decomposes at room temperature to form chlorine, oxygen, and dichlorine hexoxide
Dichlorine hexoxide is the chemical compound with the molecular formula , which is correct for its gaseous state. However, in liquid or solid form, this chlorine oxide ionizes into the dark red ionic compound chloryl perchlorate , which may be ...
(Cl2O6). Chlorine perchlorate may also be considered a chlorine derivative of perchloric acid
Perchloric acid is a mineral acid with the formula H Cl O4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous s ...
(HOClO3), similar to the thermally unstable chlorine derivatives of other oxoacids: examples include chlorine nitrate
Chlorine nitrate, with chemical formula ClNO3 is an important atmospheric gas present in the stratosphere. It is an important sink of chlorine that contributes to the depletion of ozone.
It explosively reacts with metals, metal chlorides, alcohol ...
(ClONO2, vigorously reactive and explosive), and chlorine fluorosulfate (ClOSO2F, more stable but still moisture-sensitive and highly reactive). Dichlorine hexoxide is a dark-red liquid that freezes to form a solid which turns yellow at −180 °C: it is usually made by reaction of chlorine dioxide with oxygen. Despite attempts to rationalise it as the dimer of ClO3, it reacts more as though it were chloryl perchlorate, lO2sup>+ lO4sup>−, which has been confirmed to be the correct structure of the solid. It hydrolyses in water to give a mixture of chloric and perchloric acids: the analogous reaction with anhydrous hydrogen fluoride does not proceed to completion.
Dichlorine heptoxide
Dichlorine heptoxide is the chemical compound with the formula Cl2O7. This chlorine oxide is the anhydride of perchloric acid. It is produced by the careful distillation of perchloric acid in the presence of the dehydrating agent phosphorus pent ...
(Cl2O7) is the anhydride of perchloric acid
Perchloric acid is a mineral acid with the formula H Cl O4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous s ...
(HClO4) and can readily be obtained from it by dehydrating it with phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solutio ...
at −10 °C and then distilling the product at −35 °C and 1 mmHg. It is a shock-sensitive, colourless oily liquid. It is the least reactive of the chlorine oxides, being the only one to not set organic materials on fire at room temperature. It may be dissolved in water to regenerate perchloric acid or in aqueous alkalis to regenerate perchlorates. However, it thermally decomposes explosively by breaking one of the central Cl–O bonds, producing the radicals ClO3 and ClO4 which immediately decompose to the elements through intermediate oxides.
Chlorine oxoacids and oxyanions
Chlorine forms four oxoacids:hypochlorous acid
Hypochlorous acid (HClO, HOCl, or ClHO) is a weak acid that forms when chlorine dissolves in water, and itself partially dissociates, forming hypochlorite, ClO−. HClO and ClO− are oxidizers, and the primary disinfection agents of chlorine so ...
(HOCl), chlorous acid
Chlorous acid is an inorganic compound with the formula HClO2. It is a weak acid. Chlorine has oxidation state +3 in this acid. The pure substance is unstable, disproportionating to hypochlorous acid (Cl oxidation state +1) and chloric acid (Cl ...
(HOClO), chloric acid
Chloric acid, H Cl O3, is an oxoacid of chlorine, and the formal precursor of chlorate salts. It is a strong acid ( p''K''a ≈ −2.7 (''***note: pKa not in agreement with properties in chem box at right'')) and oxidizing agent.
Properties
Chlo ...
(HOClO2), and perchloric acid
Perchloric acid is a mineral acid with the formula H Cl O4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous s ...
(HOClO3). As can be seen from the redox potentials given in the adjacent table, chlorine is much more stable towards disproportionation in acidic solutions than in alkaline solutions:
:
The hypochlorite ions also disproportionate further to produce chloride and chlorate (3 ClO− 2 Cl− + ) but this reaction is quite slow at temperatures below 70 °C in spite of the very favourable equilibrium constant of 1027. The chlorate ions may themselves disproportionate to form chloride and perchlorate (4 Cl− + 3 ) but this is still very slow even at 100 °C despite the very favourable equilibrium constant of 1020. The rates of reaction for the chlorine oxyanions increases as the oxidation state of chlorine decreases. The strengths of the chlorine oxyacids increase very quickly as the oxidation state of chlorine increases due to the increasing delocalisation of charge over more and more oxygen atoms in their conjugate bases.
Most of the chlorine oxoacids may be produced by exploiting these disproportionation reactions. Hypochlorous acid (HOCl) is highly reactive and quite unstable; its salts are mostly used for their bleaching and sterilising abilities. They are very strong oxidising agents, transferring an oxygen atom to most inorganic species. Chlorous acid (HOClO) is even more unstable and cannot be isolated or concentrated without decomposition: it is known from the decomposition of aqueous chlorine dioxide. However, sodium chlorite
Sodium chlorite (NaClO2) is a chemical compound used in the manufacturing of paper and as a disinfectant.
Use
The main application of sodium chlorite is the generation of chlorine dioxide for bleaching and stripping of textiles, pulp, and pap ...
is a stable salt and is useful for bleaching and stripping textiles, as an oxidising agent, and as a source of chlorine dioxide. Chloric acid (HOClO2) is a strong acid that is quite stable in cold water up to 30% concentration, but on warming gives chlorine and chlorine dioxide. Evaporation under reduced pressure allows it to be concentrated further to about 40%, but then it decomposes to perchloric acid, chlorine, oxygen, water, and chlorine dioxide. Its most important salt is sodium chlorate
Sodium chlorate is an inorganic compound with the chemical formula Na ClO3. It is a white crystalline powder that is readily soluble in water. It is hygroscopic. It decomposes above 300 °C to release oxygen and leaves sodium chloride. Sever ...
, mostly used to make chlorine dioxide to bleach paper pulp. The decomposition of chlorate to chloride and oxygen is a common way to produce oxygen in the laboratory on a small scale. Chloride and chlorate may comproportionate to form chlorine as follows:
: + 5 Cl− + 6 H+ ⟶ 3 Cl2 + 3 H2O
Perchlorates and perchloric acid (HOClO3) are the most stable oxo-compounds of chlorine, in keeping with the fact that chlorine compounds are most stable when the chlorine atom is in its lowest (−1) or highest (+7) possible oxidation states. Perchloric acid and aqueous perchlorates are vigorous and sometimes violent oxidising agents when heated, in stark contrast to their mostly inactive nature at room temperature due to the high activation energies for these reactions for kinetic reasons. Perchlorates are made by electrolytically oxidising sodium chlorate, and perchloric acid is made by reacting anhydrous sodium perchlorate
Sodium perchlorate is the inorganic compound with the chemical formula Na ClO4. It is a white crystalline, hygroscopic solid that is highly soluble in water and in alcohol. It is usually encountered as the monohydrate. The compound is noteworthy ...
or barium perchlorate with concentrated hydrochloric acid, filtering away the chloride precipitated and distilling the filtrate to concentrate it. Anhydrous perchloric acid is a colourless mobile liquid that is sensitive to shock that explodes on contact with most organic compounds, sets hydrogen iodide
Hydrogen iodide () is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under s ...
and thionyl chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately per year bein ...
on fire and even oxidises silver and gold. Although it is a weak ligand, weaker than water, a few compounds involving coordinated are known.
Organochlorine compounds
 Like the other carbon–halogen bonds, the C–Cl bond is a common functional group that forms part of core
Like the other carbon–halogen bonds, the C–Cl bond is a common functional group that forms part of core organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
. Formally, compounds with this functional group may be considered organic derivatives of the chloride anion. Due to the difference of electronegativity between chlorine (3.16) and carbon (2.55), the carbon in a C–Cl bond is electron-deficient and thus electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that car ...
. Chlorination modifies the physical properties of hydrocarbons in several ways: chlorocarbons are typically denser than water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
due to the higher atomic weight of chlorine versus hydrogen, and aliphatic organochloride
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine. The chloroalkane class (alkanes with one or more hydrogens substituted by chlor ...
s are alkylating agent
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting ...
s because chloride is a leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited ...
.M. Rossberg et al. "Chlorinated Hydrocarbons" in ''Ullmann's Encyclopedia of Industrial Chemistry'' 2006, Wiley-VCH, Weinheim.
Alkanes
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in whic ...
and aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
alkanes may be chlorinated under free-radical
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spon ...
conditions, with UV light. However, the extent of chlorination is difficult to control: the reaction is not regioselective
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong ba ...
and often results in a mixture of various isomers with different degrees of chlorination, though this may be permissible if the products are easily separated. Aryl chlorides may be prepared by the Friedel-Crafts halogenation, using chlorine and a Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
catalyst. The haloform reaction
In chemistry, the haloform reaction is a chemical reaction in which a haloform (, where X is a halogen) is produced by the exhaustive halogenation of an acetyl group (, where R can be either a hydrogen atom, an alkyl or an aryl group), in ...
, using chlorine and sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkal ...
, is also able to generate alkyl halides from methyl ketones, and related compounds. Chlorine adds to the multiple bonds on alkenes and alkynes as well, giving di- or tetra-chloro compounds. However, due to the expense and reactivity of chlorine, organochlorine compounds are more commonly produced by using hydrogen chloride, or with chlorinating agents such as phosphorus pentachloride
Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive and moist ...
(PCl5) or thionyl chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately per year bein ...
(SOCl2). The last is very convenient in the laboratory because all side products are gaseous and do not have to be distilled out.
Many organochlorine compounds have been isolated from natural sources ranging from bacteria to humans. Chlorinated organic compounds are found in nearly every class of biomolecules including alkaloid
Alkaloids are a class of basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Th ...
s, terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes ar ...
s, amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
s, flavonoid
Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.
Chemically, flavonoids ...
s, steroid
A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and ...
s, and fatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, ...
s. Organochlorides, including dioxins
Dioxin may refer to:
* 1,2-Dioxin or 1,4-Dioxin, two unsaturated heterocyclic 6-membered rings where two carbon atoms have been replaced by oxygen atoms, giving the molecular formula C4H4O2
* Dibenzo-1,4-dioxin, the parent compound also known ...
, are produced in the high temperature environment of forest fires, and dioxins have been found in the preserved ashes of lightning-ignited fires that predate synthetic dioxins. In addition, a variety of simple chlorinated hydrocarbons including dichloromethane, chloroform, and carbon tetrachloride
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemi ...
have been isolated from marine algae. A majority of the chloromethane
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, odorless, flammable gas. Methyl chloride is a crucial reagent in industrial ...
in the environment is produced naturally by biological decomposition, forest fires, and volcanoes.
Some types of organochlorides, though not all, have significant toxicity to plants or animals, including humans. Dioxins, produced when organic matter is burned in the presence of chlorine, and some insecticides, such as DDT
Dichlorodiphenyltrichloroethane, commonly known as DDT, is a colorless, tasteless, and almost odorless crystalline chemical compound, an organochloride. Originally developed as an insecticide, it became infamous for its environmental impacts. ...
, are persistent organic pollutant
Persistent organic pollutants (POPs), sometimes known as "forever chemicals", are organic compounds that are resistant to environmental degradation through chemical, biological, and photolytic processes. They are toxic chemicals that adverse ...
s which pose dangers when they are released into the environment. For example, DDT, which was widely used to control insects in the mid 20th century, also accumulates in food chains, and causes reproductive problems (e.g., eggshell thinning) in certain bird species. Due to the ready homolytic fission of the C–Cl bond to create chlorine radicals in the upper atmosphere, chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and pro ...
s have been phased out due to the harm they do to the ozone layer.
Occurrence and production
 Chlorine is too reactive to occur as the free element in nature but is very abundant in the form of its chloride salts. It is the twenty-first most abundant element in Earth's crust and makes up 126
Chlorine is too reactive to occur as the free element in nature but is very abundant in the form of its chloride salts. It is the twenty-first most abundant element in Earth's crust and makes up 126 parts per million
In science and engineering, the parts-per notation is a set of pseudo-units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, th ...
of it, through the large deposits of chloride minerals, especially sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35 ...
, that have been evaporated from water bodies. All of these pale in comparison to the reserves of chloride ions in seawater: smaller amounts at higher concentrations occur in some inland seas and underground brine
Brine is a high-concentration Solution (chemistry), solution of salt (NaCl) in water (H2O). In diverse contexts, ''brine'' may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of that of ...
wells, such as the Great Salt Lake in Utah and the Dead Sea
The Dead Sea ( he, יַם הַמֶּלַח, ''Yam hamMelaḥ''; ar, اَلْبَحْرُ الْمَيْتُ, ''Āl-Baḥrū l-Maytū''), also known by other names, is a salt lake bordered by Jordan to the east and Israel and the West Bank ...
in Israel.
Small batches of chlorine gas are prepared in the laboratory by combining hydrochloric acid and manganese dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cel ...
, but the need rarely arises due to its ready availability. In industry, elemental chlorine is usually produced by the electrolysis of sodium chloride dissolved in water. This method, the chloralkali process
The chloralkali process (also chlor-alkali and chlor alkali) is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide (caustic soda), which are commodit ...
industrialized in 1892, now provides most industrial chlorine gas. Along with chlorine, the method yields hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
gas and sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkal ...
, which is the most valuable product. The process proceeds according to the following chemical equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between ...
:
:2 NaCl + 2 H2O → Cl2 + H2 + 2 NaOH
The electrolysis of chloride solutions all proceed according to the following equations:
:Cathode: 2 H2O + 2 e− → H2 + 2 OH−
:Anode: 2 Cl− → Cl2 + 2 e−
In diaphragm cell electrolysis, an asbestos (or polymer-fiber) diaphragm separates a cathode and an anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemoni ...
, preventing the chlorine forming at the anode from re-mixing with the sodium hydroxide and the hydrogen formed at the cathode. The salt solution (brine) is continuously fed to the anode compartment and flows through the diaphragm to the cathode compartment, where the caustic
Caustic most commonly refers to:
* Causticity, a property of various corrosive substances
** Sodium hydroxide, sometimes called ''caustic soda''
** Potassium hydroxide, sometimes called ''caustic potash''
** Calcium oxide, sometimes called ''caust ...
alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of ...
is produced and the brine is partially depleted. Diaphragm methods produce dilute and slightly impure alkali, but they are not burdened with the problem of mercury disposal and they are more energy efficient.
Membrane cell electrolysis employs permeable membrane as an ion exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
r. Saturated sodium (or potassium) chloride solution is passed through the anode compartment, leaving at a lower concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'' ...
. This method also produces very pure sodium (or potassium) hydroxide but has the disadvantage of requiring very pure brine at high concentrations.
Deacon process
The Deacon process, invented by Henry Deacon, is a process used during the manufacture of alkalis (the initial end product was sodium carbonate) by the Leblanc process. Hydrogen chloride gas was converted to chlorine gas, which was then used to ...
, hydrogen chloride recovered from the production of organochlorine compound
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine. The chloroalkane class (alkanes with one or more hydrogens substituted by chlo ...
s is recovered as chlorine. The process relies on oxidation using oxygen:
: 4 HCl + O2 → 2 Cl2 + 2 H2O
The reaction requires a catalyst. As introduced by Deacon, early catalysts were based on copper. Commercial processes, such as the Mitsui MT-Chlorine Process, have switched to chromium and ruthenium-based catalysts.Schmittinger, Peter ''et al.'' (2006) "Chlorine" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH Verlag GmbH & Co., The chlorine produced is available in cylinders from sizes ranging from 450 g to 70 kg, as well as drums (865 kg), tank wagons (15 tonnes on roads; 27–90 tonnes by rail), and barges (600–1200 tonnes).
Applications
Sodium chloride is the most common chlorine compound, and is the main source of chlorine for the demand by the chemical industry. About 15000 chlorine-containing compounds are commercially traded, including such diverse compounds as chlorinatedmethane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ear ...
, ethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petro ...
s, vinyl chloride
Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC ...
, polyvinyl chloride (PVC), aluminium trichloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms hexahydrate with the formula , containing six water molecules of hydration. Both are colourless crystals, but samples are often contam ...
for catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycl ...
, the chlorides of magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
, titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resista ...
, zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'' ...
, and hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by D ...
which are the precursors for producing the pure form of those elements.
Quantitatively, of all elemental chlorine produced, about 63% is used in the manufacture of organic compounds, and 18% in the manufacture of inorganic chlorine compounds. About 15,000 chlorine compounds are used commercially. The remaining 19% of chlorine produced is used for bleaches and disinfection products. The most significant of organic compounds in terms of production volume are 1,2-dichloroethane
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vin ...
and vinyl chloride
Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC ...
, intermediates in the production of PVC. Other particularly important organochlorines are methyl chloride
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, odorless, flammable gas. Methyl chloride is a crucial reagent in industrial ...
, methylene chloride
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with ...
, chloroform
Chloroform, or trichloromethane, is an organic compound with formula C H Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to PTFE. It is also a precursor to various re ...
, vinylidene chloride
1,1-Dichloroethene, commonly called 1,1-dichloroethylene or vinylidene chloride or 1,1-DCE, is an organochloride with the molecular formula CHCl. It is a colorless liquid with a sharp odor. Like most chlorocarbons, it is poorly soluble in water, ...
, trichloroethylene
The chemical compound trichloroethylene is a halocarbon commonly used as an industrial solvent. It is a clear, colourless non-flammable liquid with a chloroform-like sweet smell. It should not be confused with the similar 1,1,1-trichloroethane, ...
, perchloroethylene
Tetrachloroethylene, also known under the systematic name tetrachloroethene, or perchloroethylene, and many other names (and abbreviations such as "perc" or "PERC", and "PCE"), is a chlorocarbon with the formula Cl2C=CCl2 . It is a colorless li ...
, allyl chloride
Allyl chloride is the organic compound with the formula C H2=CHCH2 Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a ch ...
, epichlorohydrin
Epichlorohydrin (abbreviated ECH) is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar orga ...
, chlorobenzene
Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.
Uses
Historical
The major use of chloro ...
, dichlorobenzene There are three distinct chemical compounds which are dichlorobenzenes:
*1,2-Dichlorobenzene or ''ortho''-dichlorobenzene;
*1,3-Dichlorobenzene or ''meta''-dichlorobenzene;
*1,4-Dichlorobenzene or ''para''-dichlorobenzene.
All three isomers are col ...
s, and trichlorobenzene
Trichlorobenzene (TCB) may refer to any of three isomeric chlorinated derivatives of benzene with the molecular formula C6H3Cl3. Trichlorobenzenes are man-made chemical compounds that occur in three different forms. Even though the forms have the ...
s. The major inorganic compounds include HCl, Cl2O, HOCl, NaClO3, chlorinated isocyanurates, AlCl3, SiCl4, SnCl4, PCl3, PCl5, POCl3, AsCl3, SbCl3, SbCl5, BiCl3, and ZnCl2.
Sanitation, disinfection, and antisepsis
Combating putrefaction
In France (as elsewhere), animal intestines were processed to make musical instrument strings,Goldbeater's skin
Goldbeater's skin is the processed outer membrane of the intestine of an animal, typically cattle, which is valued for its strength against tearing. The term derives from its traditional use as durable layers interleaved between sheets of gold st ...
and other products. This was done in "gut factories" (''boyauderies''), and it was an odiferous and unhealthy process. In or about 1820, the Société d'encouragement pour l'industrie nationale offered a prize for the discovery of a method, chemical or mechanical, for separating the peritoneal
The peritoneum is the serous membrane forming the lining of the abdominal cavity or coelom in amniotes and some invertebrates, such as annelids. It covers most of the intra-abdominal (or coelomic) organs, and is composed of a layer of mesothe ...
membrane of animal intestines without putrefaction
Putrefaction is the fifth stage of death, following pallor mortis, algor mortis, rigor mortis, and livor mortis. This process references the breaking down of a body of an animal, such as a human, post-mortem. In broad terms, it can be vie ...
. The prize was won by Antoine-Germain Labarraque, a 44-year-old French chemist and pharmacist who had discovered that Berthollet's chlorinated bleaching solutions ("''Eau de Javel
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an inorganic chemical compound with the formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may also be viewed as the sodium salt of ...
''") not only destroyed the smell of putrefaction of animal tissue decomposition, but also actually retarded the decomposition.
Labarraque's research resulted in the use of chlorides and hypochlorites of lime (calcium hypochlorite
Calcium hypochlorite is an inorganic compound with formula Ca(OCl)2. It is the main active ingredient of commercial products called bleaching powder, chlorine powder, or chlorinated lime, used for water treatment and as a bleaching agent. Th ...
) and of sodium (sodium hypochlorite
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an inorganic chemical compound with the formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may also be viewed as the sodium salt of ...
) in the ''boyauderies.'' The same chemicals were found to be useful in the routine disinfection
A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than s ...
and deodorization of latrine
A latrine is a toilet or an even simpler facility that is used as a toilet within a sanitation system. For example, it can be a communal trench in the earth in a camp to be used as emergency sanitation, a hole in the ground ( pit latrine), or ...
s, sewers, markets, abattoir
A slaughterhouse, also called abattoir (), is a facility where animals are Animal slaughter, slaughtered to provide food. Slaughterhouses supply meat, which then becomes the responsibility of a Meat packing industry, packaging facility.
Slaug ...
s, anatomical theatre
An anatomical theatre (Latin: ) was a specialised building or room, resembling a theatre, used in teaching anatomy
Anatomy () is the branch of biology concerned with the study of the structure of organisms and their parts. Anatomy is a b ...
s, and morgues. They were successful in hospitals, lazarets, prison
A prison, also known as a jail, gaol (dated, standard English, Australian, and historically in Canada), penitentiary (American English and Canadian English), detention center (or detention centre outside the US), correction center, correc ...
s, infirmaries (both on land and at sea), magnaneries, stables, cattle-sheds, etc.; and they were beneficial during exhumation
Burial, also known as interment or inhumation, is a method of final disposition whereby a dead body is placed into the ground, sometimes with objects. This is usually accomplished by excavating a pit or trench, placing the deceased and objec ...
s, embalming
Embalming is the art and science of preserving human remains by treating them (in its modern form with chemicals) to forestall decomposition. This is usually done to make the deceased suitable for public or private viewing as part of the funeral ...
, outbreaks of epidemic disease, fever, and blackleg in cattle.
Disinfection
Labarraque's chlorinated lime and soda solutions have been advocated since 1828 to prevent infection (called "contagious infection", presumed to be transmitted by " miasmas"), and to treatputrefaction
Putrefaction is the fifth stage of death, following pallor mortis, algor mortis, rigor mortis, and livor mortis. This process references the breaking down of a body of an animal, such as a human, post-mortem. In broad terms, it can be vie ...
of existing wounds, including septic wounds. In his 1828 work, Labarraque recommended that doctors breathe chlorine, wash their hands in chlorinated lime, and even sprinkle chlorinated lime about the patients' beds in cases of "contagious infection". In 1828, the contagion of infections was well known, even though the agency of the microbe
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
was not discovered until more than half a century later.
During the Paris cholera outbreak of 1832, large quantities of so-called ''chloride of lime'' were used to disinfect the capital. This was not simply modern calcium chloride
Calcium chloride is an inorganic compound, a salt with the chemical formula . It is a white crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with calcium hydroxide.
Cal ...
, but chlorine gas dissolved in lime-water (dilute calcium hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed or slaked with water. It has m ...
) to form calcium hypochlorite
Calcium hypochlorite is an inorganic compound with formula Ca(OCl)2. It is the main active ingredient of commercial products called bleaching powder, chlorine powder, or chlorinated lime, used for water treatment and as a bleaching agent. Th ...
(chlorinated lime). Labarraque's discovery helped to remove the terrible stench of decay from hospitals and dissecting rooms, and by doing so, effectively deodorised the Latin Quarter
The Latin Quarter of Paris (french: Quartier latin, ) is an area in the 5th and the 6th arrondissements of Paris. It is situated on the left bank of the Seine, around the Sorbonne.
Known for its student life, lively atmosphere, and bistros, ...
of Paris.Corbin, Alain (1988). The Foul and the Fragrant: Odor and the French Social Imagination
''. Harvard University Press. pp. 121–22. These "putrid miasmas" were thought by many to cause the spread of "contagion" and "infection" – both words used before the germ theory of infection. Chloride of lime was used for destroying odors and "putrid matter". One source claims chloride of lime was used by Dr. John Snow to disinfect water from the cholera-contaminated well that was feeding the Broad Street pump in 1854 London, though three other reputable sources that describe that famous cholera epidemic do not mention the incident.Vinten-Johansen, Peter, Howard Brody, Nigel Paneth, Stephen Rachman and Michael Rip. (2003). ''Cholera, Chloroform, and the Science of Medicine''. New York:Oxford University. One reference makes it clear that chloride of lime was used to disinfect the
offal
Offal (), also called variety meats, pluck or organ meats, is the organs of a butchered animal. The word does not refer to a particular list of edible organs, which varies by culture and region, but usually excludes muscle. Offal may also ref ...
and filth in the streets surrounding the Broad Street pump – a common practice in mid-nineteenth century England.
Semmelweis and experiments with antisepsis
 Perhaps the most famous application of Labarraque's chlorine and chemical base solutions was in 1847, when
Perhaps the most famous application of Labarraque's chlorine and chemical base solutions was in 1847, when Ignaz Semmelweis
Ignaz Philipp Semmelweis (; hu, Semmelweis Ignác Fülöp ; 1 July 1818 – 13 August 1865) was a Hungarian physician and scientist, who was an early pioneer of antiseptic procedures. Described as the "saviour of mothers", he discovered that t ...
used chlorine-water (chlorine dissolved in pure water, which was cheaper than chlorinated lime solutions) to disinfect the hands of Austrian doctors, which Semmelweis noticed still carried the stench of decomposition from the dissection rooms to the patient examination rooms. Long before the germ theory of disease, Semmelweis theorized that "cadaveric particles" were transmitting decay from fresh medical cadavers to living patients, and he used the well-known "Labarraque's solutions" as the only known method to remove the smell of decay and tissue decomposition (which he found that soap did not). The solutions proved to be far more effective antiseptics than soap (Semmelweis was also aware of their greater efficacy, but not the reason), and this resulted in Semmelweis's celebrated success in stopping the transmission of childbed fever ("puerperal fever") in the maternity wards of Vienna General Hospital
The Vienna General Hospital (german: Allgemeines Krankenhaus der Stadt Wien), usually abbreviated to AKH, is the general hospital of the city of Vienna, Austria. It is also the city's university hospital, and the site of the Medical Univer ...
in Austria
Austria, , bar, Östareich officially the Republic of Austria, is a country in the southern part of Central Europe, lying in the Eastern Alps. It is a federation of nine states, one of which is the capital, Vienna, the most populous ...
in 1847.
Much later, during World War I in 1916, a standardized and diluted modification of Labarraque's solution containing hypochlorite (0.5%) and boric acid as an acidic stabilizer was developed by Henry Drysdale Dakin (who gave full credit to Labarraque's prior work in this area). Called Dakin's solution Dakin's solution is a dilute solution of sodium hypochlorite (0.4% to 0.5%) and other stabilizing ingredients, traditionally used as an antiseptic, e.g. to cleanse wounds in order to prevent infection.Jeffrey M. Levine (2013): "Dakin’s Solution: P ...
, the method of wound irrigation with chlorinated solutions allowed antiseptic treatment of a wide variety of open wounds, long before the modern antibiotic era. A modified version of this solution continues to be employed in wound irrigation in modern times, where it remains effective against bacteria that are resistant to multiple antibiotics (see Century Pharmaceuticals).
Public sanitation
 The first continuous application of chlorination to drinking U.S. water was installed in
The first continuous application of chlorination to drinking U.S. water was installed in Jersey City
Jersey City is the second-most populous city (New Jersey), city in the U.S. state of New Jersey, after Newark, New Jersey, Newark.
, New Jersey, in 1908. By 1918, the US Department of Treasury called for all drinking water to be disinfected with chlorine. Chlorine is presently an important chemical for water purification
Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from water. The goal is to produce water that is fit for specific purposes. Most water is purified and disinfected for hu ...
(such as in water treatment plants), in disinfectant
A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than s ...
s, and in bleach. Even small water supplies are now routinely chlorinated.
Chlorine is usually used (in the form of hypochlorous acid
Hypochlorous acid (HClO, HOCl, or ClHO) is a weak acid that forms when chlorine dissolves in water, and itself partially dissociates, forming hypochlorite, ClO−. HClO and ClO− are oxidizers, and the primary disinfection agents of chlorine so ...
) to kill bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were am ...
and other microbes in drinking water
Drinking water is water that is used in drink or food preparation; potable water is water that is safe to be used as drinking water. The amount of drinking water required to maintain good health varies, and depends on physical activity level, ...
supplies and public swimming pools. In most private swimming pools, chlorine itself is not used, but rather sodium hypochlorite
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an inorganic chemical compound with the formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may also be viewed as the sodium salt of ...
, formed from chlorine and sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkal ...
, or solid tablets of chlorinated isocyanurates. The drawback of using chlorine in swimming pools is that the chlorine reacts with the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
s in proteins in human hair and skin. Contrary to popular belief, the distinctive "chlorine aroma" associated with swimming pools is not the result of elemental chlorine itself, but of chloramine
Chloramines refer to derivatives of ammonia and organic amines wherein one or more N-H bonds have been replaced by N-Cl bonds. Two classes of compounds are considered: inorganic chloramines and organic chloramines.
Inorganic chloramines
Inorgan ...
, a chemical compound produced by the reaction of free dissolved chlorine with amines in organic substances including those in urine and sweat. As a disinfectant in water, chlorine is more than three times as effective against ''Escherichia coli'' as bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simil ...
, and more than six times as effective as iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
. Increasingly, monochloramine itself is being directly added to drinking water for purposes of disinfection, a process known as chloramination.
It is often impractical to store and use poisonous chlorine gas for water treatment, so alternative methods of adding chlorine are used. These include hypochlorite
In chemistry, hypochlorite is an anion with the chemical formula ClO−. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite (household bleach) and calcium hypochlorite (a component of ble ...
solutions, which gradually release chlorine into the water, and compounds like sodium dichloro-s-triazinetrione (dihydrate or anhydrous), sometimes referred to as "dichlor", and trichloro-s-triazinetrione, sometimes referred to as "trichlor". These compounds are stable while solid and may be used in powdered, granular, or tablet form. When added in small amounts to pool water or industrial water systems, the chlorine atoms hydrolyze from the rest of the molecule, forming hypochlorous acid (HOCl), which acts as a general biocide, killing germs, microorganisms, algae, and so on.
Use as a weapon
World War I
Chlorine gas, also known as bertholite, was first chemical warfare, used as a weapon in World War I by Germany on April 22, 1915, in the Second Battle of Ypres. As described by the soldiers, it had the distinctive smell of a mixture of pepper and pineapple. It also tasted metallic and stung the back of the throat and chest. Chlorine reacts with water in the Mucous membrane, mucosa of the lungs to formhydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
, destructive to living tissue and potentially lethal. Human respiratory systems can be protected from chlorine gas by gas masks with activated charcoal or other filters, which makes chlorine gas much less lethal than other chemical weapons. It was pioneered by a German scientist later to be a Nobel laureate, Fritz Haber of the Kaiser Wilhelm Institute for Chemistry, Kaiser Wilhelm Institute in Berlin, in collaboration with the German chemical conglomerate IG Farben, which developed methods for discharging chlorine gas against an trench, entrenched enemy. After its first use, both sides in the conflict used chlorine as a chemical weapon, but it was soon replaced by the more deadly phosgene and mustard gas.
Middle east
Chlorine gas was also used during the Iraq War in Anbar Province in 2007, with insurgents packing truck bombs with mortar (weapon), mortar shells and chlorine tanks. The attacks killed two people from the explosives and sickened more than 350. Most of the deaths were caused by the force of the explosions rather than the effects of chlorine since the toxic gas is readily dispersed and diluted in the atmosphere by the blast. In some bombings, over a hundred civilians were hospitalized due to breathing difficulties. The Iraqi authorities tightened security for elemental chlorine, which is essential for providing safe drinking water to the population. On 23 October 2014, it was reported that the Islamic State of Iraq and the Levant had used chlorine gas in the town of Duluiyah, Iraq. Laboratory analysis of clothing and soil samples confirmed the use of chlorine gas against Kurdish Peshmerga Forces in a vehicle-borne improvised explosive device attack on 23 January 2015 at the Highway 47 Kiske Junction near Mosul. Another country in the middle east, Syria, has used chlorine as a chemical weapon delivered from barrel bombs and rockets. In 2016, the OPCW-UN Joint Investigative Mechanism concluded that the Syrian government used chlorine as a chemical weapon in three separate attacks. Later investigations from the OPCW's Investigation and Identification Team concluded that the Syrian Air Force was responsible for chlorine attacks in 2017 and 2018.Biological role
Thechloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride s ...
anion is an Mineral (nutrient), essential nutrient for metabolism. Chlorine is needed for the production of hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
in the stomach and in cellular pump functions. The main dietary source is table salt, or sodium chloride. Overly low or high concentrations of chloride in the blood are examples of electrolyte disturbances. Hypochloremia (having too little chloride) rarely occurs in the absence of other abnormalities. It is sometimes associated with hypoventilation. It can be associated with chronic respiratory acidosis. Hyperchloremia (having too much chloride) usually does not produce symptoms. When symptoms do occur, they tend to resemble those of hypernatremia (having too much sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
). Reduction in blood chloride leads to cerebral dehydration; symptoms are most often caused by rapid rehydration which results in cerebral edema. Hyperchloremia can affect oxygen transport.
Hazards
Chlorine is a toxic gas that attacks the respiratory system, eyes, and skin. Because it is denser than air, it tends to accumulate at the bottom of poorly ventilated spaces. Chlorine gas is a strong oxidizer, which may react with flammable materials. Chlorine is detectable with measuring devices in concentrations as low as 0.2 parts per million (ppm), and by smell at 3 ppm. Coughing and vomiting may occur at 30 ppm and lung damage at 60 ppm. About 1000 ppm can be fatal after a few deep breaths of the gas. The IDLH (immediately dangerous to life and health) concentration is 10 ppm. Breathing lower concentrations can aggravate the respiratory system and exposure to the gas can irritate the eyes. When chlorine is inhaled at concentrations greater than 30 ppm, it reacts with water within the lungs, producinghydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
(HCl) and hypochlorous acid
Hypochlorous acid (HClO, HOCl, or ClHO) is a weak acid that forms when chlorine dissolves in water, and itself partially dissociates, forming hypochlorite, ClO−. HClO and ClO− are oxidizers, and the primary disinfection agents of chlorine so ...
(HClO).
When used at specified levels for water disinfection, the reaction of chlorine with water is not a major concern for human health. Other materials present in the water may generate disinfection by-products that are associated with negative effects on human health.
In the United States, the Occupational Safety and Health Administration (OSHA) has set the permissible exposure limit for elemental chlorine at 1 ppm, or 3 mg/m3. The National Institute for Occupational Safety and Health has designated a recommended exposure limit of 0.5 ppm over 15 minutes.
In the home, accidents occur when hypochlorite bleach solutions come into contact with certain acidic drain-cleaners to produce chlorine gas. Hypochlorite bleach (a popular laundry additive) combined with ammonia (another popular laundry additive) produces chloramines, another toxic group of chemicals.
Chlorine-induced cracking in structural materials
Chlorine is widely used for purifying water, especially potable water supplies and water used in swimming pools. Several catastrophic collapses of swimming pool ceilings have occurred from chlorine-induced stress corrosion cracking of stainless steel suspension rods. Some polymers are also sensitive to attack, including acetal resin and polybutene. Both materials were used in hot and cold water domestic plumbing, and stress corrosion cracking caused widespread failures in the US in the 1980s and 1990s.
Chlorine-iron fire
The elementiron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
can combine with chlorine at high temperatures in a strong exothermic reaction, creating a ''chlorine-iron fire''. Chlorine-iron fires are a risk in chemical process plants, where much of the pipework that carries chlorine gas is made of steel.
See also
* Chlorine cycle * Chlorine gas poisoning * Industrial gas * Polymer degradation * Reductive dechlorinationReferences
Explanatory notes
General bibliography
*External links
Chlorine
at ''The Periodic Table of Videos'' (University of Nottingham) * Agency for Toxic Substances and Disease Registry
Chlorine
Chlorine Production Using Mercury, Environmental Considerations and Alternatives
National Institute for Occupational Safety and Health – Chlorine Page
Chlorine Institute
– Trade association representing the chlorine industry
Chlorine Online
– the web portal of Eurochlor – the business association of the European chlor-alkali industry * {{Authority control Chlorine, Chemical elements Diatomic nonmetals Gases with color Halogens Hazardous air pollutants Industrial gases Occupational safety and health Oxidizing agents Pulmonary agents Reactive nonmetals Swimming pool equipment World Health Organization essential medicines