|
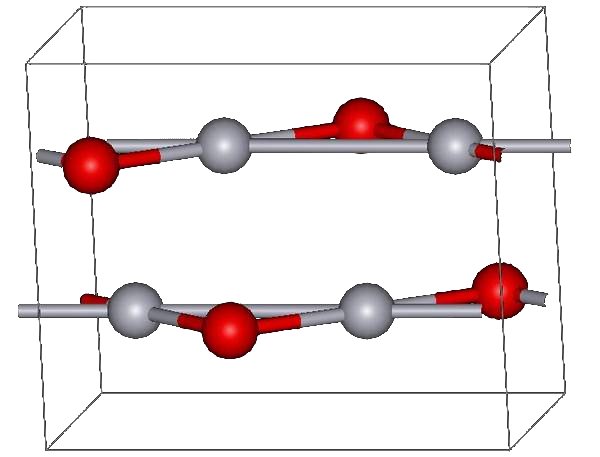
Mercury(II) Oxide
Mercury(II) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula Hg O. It has a red or orange color. Mercury(II) oxide is a solid at room temperature and pressure. The mineral form montroydite is very rarely found. History An experiment for the preparation of mercuric oxide was first described by 11th century Arab-Spanish alchemist, Maslama al-Majriti, in ''Rutbat al-hakim.'' In 1774, Joseph Priestley discovered that oxygen was released by heating mercuric oxide, although he did not identify the gas as oxygen (rather, Priestley called it " dephlogisticated air," as that was the paradigm that he was working under at the time). Synthesis The red form of HgO can be made by heating Hg in oxygen at roughly 350 °C, or by pyrolysis of Hg(NO3)2. The yellow form can be obtained by precipitation of aqueous Hg2+ with alkali. The difference in color is due to particle size; both forms have the same structure consisting of near ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Montroydite
Montroydite is the mineral form of mercury(II) oxide with formula HgO. It is a rare mercury mineral. It was first described for an occurrence in the mercury deposit at Terlingua, Texas and named for Montroyd Sharp who was an owner of the deposit. Montroydite occurs in mercury deposits of hydrothermal origin. Associated minerals include: native mercury, cinnabar, metacinnabar, calomel, eglestonite, terlinguaite, mosesite, kleinite, edgarbaileyite, gypsum, calcite Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on scra ... and dolomite. References Mercury(II) minerals Oxide minerals Orthorhombic minerals {{oxide-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver and was formerly named hydrargyrum ( ) from the Greek words, ''hydor'' (water) and ''argyros'' (silver). A heavy, silvery d-block element, mercury is the only metallic element that is known to be liquid at standard temperature and pressure; the only other element that is liquid under these conditions is the halogen bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature. Mercury occurs in deposits throughout the world mostly as cinnabar ( mercuric sulfide). The red pigment vermilion is obtained by grinding natural cinnabar or synthetic mercuric sulfide. Mercury is used in thermometers, barometers, manometers, sphygmomanometers, float valves, mercury switches, mercury relays, fluorescent lamps and other devices, though concerns about the element's toxicity have led to mercury thermometers and sphygmomanometers being largely p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The base-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexagonal Crystal System
In crystallography, the hexagonal crystal family is one of the six crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal system and the rhombohedral lattice system are not equivalent (see section crystal systems below). In particular, there are crystals that have trigonal symmetry but belong to the hexagonal lattice (such as α- quartz). The hexagonal crystal family consists of the 12 point groups such that at least one of their space groups has the hexagonal lattice as underlying lattice, and is the union of the hexagonal crystal system and the trigonal crystal system. There are 52 space groups associated with it, which are exactly those whose Bravais lattice is either hexagonal or rhombohedral. __TOC__ Lattice systems The hexagonal crystal family consists of two lattice systems: hexagonal and rhombohedral. Each lattice system consists of one Brav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnabar
Cinnabar (), or cinnabarite (), from the grc, κιννάβαρι (), is the bright scarlet to brick-red form of mercury(II) sulfide (HgS). It is the most common source ore for refining elemental mercury and is the historic source for the brilliant red or scarlet pigment termed vermilion and associated red mercury pigments. Cinnabar generally occurs as a vein-filling mineral associated with recent volcanic activity and alkaline hot springs. The mineral resembles quartz in symmetry and in its exhibiting birefringence. Cinnabar has a mean refractive index near 3.2, a hardness between 2.0 and 2.5, and a specific gravity of approximately 8.1. The color and properties derive from a structure that is a hexagonal crystalline lattice belonging to the trigonal crystal system, crystals that sometimes exhibit twinning. Cinnabar has been used for its color since antiquity in the Near East, including as a rouge-type cosmetic, in the New World since the Olmec culture, and in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base (''a'' by ''b'') and height (''c''), such that ''a'', ''b'', and ''c'' are distinct. All three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal. Bravais lattices There are four orthorhombic Bravais lattices: primitive orthorhombic, base-centered orthorhombic, body-centered orthorhombic, and face-centered orthorhombic. For the base-centered orthorhombic lattice, the primitive cell has the shape of a right rhombic prism;See , row oC, column Primitive, where the cell parameters are given as a1 = a2, α = β = 90° it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the primi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(II) Nitrate
Mercury(II) nitrate is an inorganic compound with the formula Hg(NO3)2.xH2O. These colorless or white soluble crystalline salts are occasionally used as a reagent. It is made by treating mercury with hot concentrated nitric acid. Neither anhydrous nor monohydrate has been confirmed by X-ray crystallography. The anhydrous material is more widely used. Uses Mercuric nitrate has been used in mercuration of ketones. Mercuric nitrate was formerly used in carroting felt for hats. Health information Mercury compounds are highly toxic. The use of this compound by hatters and the subsequent mercury poisoning of said hatters is a common theory of where the phrase "mad as a hatter" came from. See also * The Hatter * Mercury poisoning * Gilding References External links ATSDR - Toxic Substances Portal - Mercury(11/14/2013) ATSDR - Public Health Statement: Mercury(11/14/2013) (link not traceable 11/14/2013) ATSDR - Medical Management Guidelines for Mercury(11/14/2013) ATSDR - Tox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''pyro'' "fire", "heat", "fever" and ''lysis'' "separating". Pyrolysis is most commonly used in the treatment of organic materials. It is one of the processes involved in charring wood.''Burning of wood'' , InnoFireWood's website. Accessed on 2010-02-06. In general, pyrolysis of organic substances produces volatile products and leaves char, a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly |
Cinnabar Structure
Cinnabar (), or cinnabarite (), from the grc, κιννάβαρι (), is the bright scarlet to brick-red form of mercury(II) sulfide (HgS). It is the most common source ore for refining elemental mercury and is the historic source for the brilliant red or scarlet pigment termed vermilion and associated red mercury pigments. Cinnabar generally occurs as a vein-filling mineral associated with recent volcanic activity and alkaline hot springs. The mineral resembles quartz in symmetry and in its exhibiting birefringence. Cinnabar has a mean refractive index near 3.2, a hardness between 2.0 and 2.5, and a specific gravity of approximately 8.1. The color and properties derive from a structure that is a hexagonal crystalline lattice belonging to the trigonal crystal system, crystals that sometimes exhibit twinning. Cinnabar has been used for its color since antiquity in the Near East, including as a rouge-type cosmetic, in the New World since the Olmec culture, and in China since ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paradigm
In science and philosophy, a paradigm () is a distinct set of concepts or thought patterns, including theories, research methods, postulates, and standards for what constitute legitimate contributions to a field. Etymology ''Paradigm'' comes from Greek παράδειγμα (''paradeigma''), "pattern, example, sample" from the verb παραδείκνυμι (''paradeiknumi''), "exhibit, represent, expose" and that from παρά (''para''), "beside, beyond" and δείκνυμι (''deiknumi''), "to show, to point out". In classical (Greek-based) rhetoric, a paradeigma aims to provide an audience with an illustration of a similar occurrence. This illustration is not meant to take the audience to a conclusion, however it is used to help guide them get there. One way of how a ''paradeigma'' is meant to guide an audience would be exemplified by the role of a personal accountant. It is not the job of a personal accountant to tell a client exactly what (and what not) to spend money ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phlogiston
The phlogiston theory is a superseded scientific theory that postulated the existence of a fire-like element called phlogiston () contained within combustible bodies and released during combustion. The name comes from the Ancient Greek (''burning up''), from (''flame''). The idea was first proposed in 1667 by Johann Joachim Becher and later put together more formally by Georg Ernst Stahl. Phlogiston theory attempted to explain chemical processes such as combustion and rusting, now collectively known as oxidation. It was challenged by the concomitant weight increase, and was abandoned before the end of the 18th century following experiments by Antoine Lavoisier and others. Phlogiston theory led to experiments which ultimately concluded with the discovery of oxygen. Theory Phlogiston theory states that ''phlogisticated'' substances contain phlogiston and that they ''dephlogisticate'' when burned, releasing stored phlogiston which is absorbed by the air. Growing plants then ab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted experiments in electricity and other areas of science. He was a close friend of, and worked in close association with Benjamin Franklin involving electricity experiments. Priestley is credited with his independent discovery of oxygen by the thermal decomposition of mercuric oxide, having isolated it in 1774. During his lifetime, Priestley's considerable scientific reputation rested on his invention of carbonated water, his writings on electricity, and his discovery of several "airs" (gases), the most famous being what Priestley dubbed "dephlogisticated air" (oxygen). Priestley's determination to defend phlogiston theory and to reject what would become the chemical revolution eventually left him isolated within the scientific community. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |