Cerium Zinc on:
[Wikipedia]
[Google]
[Amazon]
Cerium is a
 Four allotropic forms of cerium are known to exist at standard pressure, and are given the common labels of α to δ:
* The high-temperature form, δ-cerium, has a bcc ( body-centered cubic) crystal structure and exists above 726 °C.
* The stable form below 726 °C to approximately room temperature is γ-cerium, with an fcc ( face-centered cubic) crystal structure.
* The DHCP (double
Four allotropic forms of cerium are known to exist at standard pressure, and are given the common labels of α to δ:
* The high-temperature form, δ-cerium, has a bcc ( body-centered cubic) crystal structure and exists above 726 °C.
* The stable form below 726 °C to approximately room temperature is γ-cerium, with an fcc ( face-centered cubic) crystal structure.
* The DHCP (double
 The compound ceric ammonium nitrate ("CAN") is the most common cerium compound encountered in the laboratory. The six nitrate ligands bind as
The compound ceric ammonium nitrate ("CAN") is the most common cerium compound encountered in the laboratory. The six nitrate ligands bind as 
 Due to ligand-to-metal charge transfer, aqueous cerium(IV) ions are orange-yellow. Aqueous cerium(IV) is metastable in water and is a strong oxidizing agent that oxidizes
Due to ligand-to-metal charge transfer, aqueous cerium(IV) ions are orange-yellow. Aqueous cerium(IV) is metastable in water and is a strong oxidizing agent that oxidizes

 Cerium was discovered in
Cerium was discovered in
 Bastnäsite, LnIIICO3F, is usually lacking in
Bastnäsite, LnIIICO3F, is usually lacking in
 Cerium has two main applications, both of which use CeO2. The industrial application of ceria is for polishing, especially
Cerium has two main applications, both of which use CeO2. The industrial application of ceria is for polishing, especially
. nanopartikel.info (2011-02-02) The most commonly used example is cerium(III)-doped yttrium aluminium garnet (Ce:YAG) which emits green to yellow-green light (550–530 nm) and also behaves as a
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Ce and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
58. Cerium is a soft, ductile, and silvery-white metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
that tarnishes when exposed to air. Cerium is the second element in the lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttr ...
series, and while it often shows the +3 oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
characteristic of the series, it also has a stable +4 state that does not oxidize water. It is also considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure.
Despite always occurring in combination with the other rare-earth elements in minerals such as those of the monazite and bastnäsite groups, cerium is easy to extract from its ores, as it can be distinguished among the lanthanides by its unique ability to be oxidized to the +4 state in aqueous solution. It is the most common of the lanthanides, followed by neodymium
Neodymium is a chemical element with the symbol Nd and atomic number 60. It is the fourth member of the lanthanide series and is considered to be one of the rare-earth metals. It is a hard, slightly malleable, silvery metal that quickly tarnishes i ...
, lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lantha ...
, and praseodymium. It is the 25th-most abundant element, making up 66 ppm of the Earth's crust, half as much as chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
and five times as much as lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
.
Cerium was the first of the lanthanides to be discovered, in Bastnäs
Bastnäs ( sv, Bastnäs or ) is an ore field near Riddarhyttan, Västmanland, Sweden. The mines in Bastnäs were earliest mentioned in 1692. Iron, copper and rare-earth elements were extracted from the mines and 4,500 tons of cerium was produced be ...
, Sweden, by Jöns Jakob Berzelius
Jöns is a Swedish given name and a surname.
Notable people with the given name include:
* Jöns Jacob Berzelius (1779–1848), Swedish chemist
* Jöns Budde (1435–1495), Franciscan friar from the Brigittine monastery in NaantaliVallis Gratiae ...
and Wilhelm Hisinger in 1803, and independently by Martin Heinrich Klaproth in Germany in the same year. In 1839 Carl Gustaf Mosander became the first to isolate the metal. Today, cerium and its compounds have a variety of uses: for example, cerium(IV) oxide is used to polish glass and is an important part of catalytic converter
A catalytic converter is an exhaust emission control device that converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less-toxic pollutants by catalyzing a redox reaction. Catalytic converters are usually ...
s. Cerium metal is used in ferrocerium
Ferrocerium (also known in Europe as Auermetall) is a synthetic pyrophoric alloy of mischmetal (cerium, lanthanum, neodymium, other trace lanthanides and some iron – about 95% lanthanides and 5% iron) hardened by blending in oxides of ...
lighters for its pyrophoric
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolith ...
properties. Cerium-doped YAG phosphor is used in conjunction with blue light-emitting diode
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (cor ...
s to produce white light in most commercial white LED light sources.
Characteristics
Physical
Cerium is the second element of thelanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttr ...
series. In the periodic table, it appears between the lanthanides lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lantha ...
to its left and praseodymium to its right, and above the actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The inform ...
thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high me ...
. It is a ductile metal with a hardness similar to that of silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
. Its 58 electrons are arranged in the configuration
Configuration or configurations may refer to:
Computing
* Computer configuration or system configuration
* Configuration file, a software file used to configure the initial settings for a computer program
* Configurator, also known as choice board ...
ef15d16s2, of which the four outer electrons are valence electrons.Greenwood and Earnshaw, pp. 1232–5 The 4f, 5d, and 6s energy levels are very close to each other, and the transfer of one electron to the 5d shell is due to strong interelectronic repulsion in the compact 4f shell. This effect is overwhelmed when the atom is positively ionised; thus Ce2+ on its own has instead the regular configuration ef2, although in some solid solutions it may be ef15d1. Most lanthanides can use only three electrons as valence electrons, as afterwards the remaining 4f electrons are too strongly bound: cerium is an exception because of the stability of the empty f-shell in Ce4+ and the fact that it comes very early in the lanthanide series, where the nuclear charge is still low enough until neodymium
Neodymium is a chemical element with the symbol Nd and atomic number 60. It is the fourth member of the lanthanide series and is considered to be one of the rare-earth metals. It is a hard, slightly malleable, silvery metal that quickly tarnishes i ...
to allow the removal of the fourth valence electron by chemical means.
Cerium has a variable electronic structure. The energy of the 4f electron is nearly the same as that of the outer 5d and 6s electrons that are delocalized in the metallic state, and only a small amount of energy is required to change the relative occupancy of these electronic levels. This gives rise to dual valence states. For example, a volume change of about 10% occurs when cerium is subjected to high pressures or low temperatures. It appears that the valence changes from about 3 to 4 when it is cooled or compressed.
Chemical properties of the element
With ''E''⦵ of −2.34 V for the Ce3+/Ce) couple, cerium metal is a good reductant.Greenwood and Earnshaw, pp. 1244–8 Logically, it tarnishes in air, forming a passivating oxide layer likeiron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in f ...
rust. A centimeter-sized sample of cerium metal corrodes completely in about a year. More dramatically, metallic cerium can be highly pyrophoric
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolith ...
:Ce + O2 → CeO2
Being highly electropositive, cerium reacts with water. The reaction is slow with cold water but speeds up with increasing temperature, producing cerium(III) hydroxide and hydrogen gas:
:2 Ce + 6 H2O → 2 Ce(OH)3 + 3 H2
Allotropes
 Four allotropic forms of cerium are known to exist at standard pressure, and are given the common labels of α to δ:
* The high-temperature form, δ-cerium, has a bcc ( body-centered cubic) crystal structure and exists above 726 °C.
* The stable form below 726 °C to approximately room temperature is γ-cerium, with an fcc ( face-centered cubic) crystal structure.
* The DHCP (double
Four allotropic forms of cerium are known to exist at standard pressure, and are given the common labels of α to δ:
* The high-temperature form, δ-cerium, has a bcc ( body-centered cubic) crystal structure and exists above 726 °C.
* The stable form below 726 °C to approximately room temperature is γ-cerium, with an fcc ( face-centered cubic) crystal structure.
* The DHCP (double hexagonal close-packed
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occu ...
) form β-cerium is the equilibrium structure approximately from room temperature to −150 °C.
* The fcc form α-cerium is stable below about −150 °C; it has a density of 8.16 g/cm3.
* Other solid phases occurring only at high pressures are shown on the phase diagram.
* Both γ and β forms are quite stable at room temperature, although the equilibrium transformation temperature is estimated at 75 °C.
At lower temperatures the behavior of cerium is complicated by the slow rates of transformation. Transformation temperatures are subject to substantial hysteresis and values quoted here are approximate. Upon cooling below −15 °C, γ-cerium starts to change to β-cerium, but the transformation involves a volume increase and, as more β forms, the internal stresses build up and suppress further transformation. Cooling below approximately −160 °C will start formation of α-cerium but this is only from remaining γ-cerium. β-cerium does not significantly transform to α-cerium except in the presence of stress or deformation. At atmospheric pressure, liquid cerium is more dense than its solid form at the melting
point.
Isotopes
Naturally occurring cerium is made up of four isotopes: 136Ce (0.19%), 138Ce (0.25%), 140Ce (88.4%), and 142Ce (11.1%). All four are observationally stable, though the light isotopes 136Ce and 138Ce are theoretically expected to undergo inverse double beta decay to isotopes ofbarium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
, and the heaviest isotope 142Ce is expected to undergo double beta decay to 142Nd or alpha decay to 138Ba. Additionally, 140Ce would release energy upon spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakdo ...
. None of these decay modes have yet been observed, though the double beta decay of 136Ce, 138Ce, and 142Ce have been experimentally searched for. The current experimental limits for their half-lives are:
:136Ce: >3.8×1016 y
:138Ce: >5.7×1016 y
:142Ce: >5.0×1016 y
All other cerium isotopes are synthetic Synthetic things are composed of multiple parts, often with the implication that they are artificial. In particular, 'synthetic' may refer to:
Science
* Synthetic chemical or compound, produced by the process of chemical synthesis
* Synthetic o ...
and radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
. The most stable of them are 144Ce with a half-life of 284.9 days, 139Ce with a half-life of 137.6 days, and 141Ce with a half-life of 32.5 days. All other radioactive cerium isotopes have half-lives under four days, and most of them have half-lives under ten minutes. The isotopes between 140Ce and 144Ce inclusive occur as fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
s of uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
. The primary decay mode of the isotopes lighter than 140Ce is inverse beta decay or electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. Thi ...
to isotopes of lanthanum
Naturally occurring lanthanum (57La) is composed of one stable (139La) and one radioactive (138La) isotope, with the stable isotope, 139La, being the most abundant (99.91% natural abundance). There are 38 radioisotopes that have been characterize ...
, while that of the heavier isotopes is beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
to isotopes of praseodymium
Naturally occurring praseodymium (59Pr) is composed of one stable isotope, 141Pr. Thirty-eight radioisotopes have been characterized with the most stable being 143Pr, with a half-life of 13.57 days and 142Pr, with a half-life of 19.12 hours. All o ...
. Some isotopes of neodymium
Naturally occurring neodymium (60Nd) is composed of 5 stable isotopes, 142Nd, 143Nd, 145Nd, 146Nd and 148Nd, with 142Nd being the most abundant (27.2% natural abundance), and 2 long-lived radioisotopes, 144Nd and 150Nd. In all, 33 radioisotopes of ...
can alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an atom ...
or are predicted to decay to isotopes of cerium.
The rarity of the proton-rich 136Ce and 138Ce is explained by the fact that they cannot be made in the most common processes of stellar nucleosynthesis
Stellar nucleosynthesis is the creation (nucleosynthesis) of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a ...
for elements beyond iron, the s-process
The slow neutron-capture process, or ''s''-process, is a series of reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The ''s''-process is responsible for the creation (nucleosynthesis) of approximat ...
(slow neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, ...
) and the r-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for the creation of approximately half of the atomic nuclei heavier than iron, the "heavy elements", ...
(rapid neutron capture). This is so because they are bypassed by the reaction flow of the s-process, and the r-process nuclides are blocked from decaying to them by more neutron-rich stable nuclides. Such nuclei are called p-nuclei, and their origin is not yet well understood: some speculated mechanisms for their formation include proton capture as well as photodisintegration. 140Ce is the most common isotope of cerium, as it can be produced in both the s- and r-processes, while 142Ce can only be produced in the r-process. Another reason for the abundance of 140Ce is that it is a magic nucleus
In nuclear physics, a magic number is a number of nucleons (either protons or neutrons, separately) such that they are arranged into complete Nuclear shell model, shells within the atomic nucleus. As a result, atomic nuclei with a 'magic' number o ...
, having a closed neutron shell (it has 82 neutrons), and hence it has a very low cross section
Cross section may refer to:
* Cross section (geometry)
** Cross-sectional views in architecture & engineering 3D
*Cross section (geology)
* Cross section (electronics)
* Radar cross section, measure of detectability
* Cross section (physics)
**Abs ...
towards further neutron capture. Although its proton number of 58 is not magic, it is granted additional stability, as its eight additional protons past the magic number 50 enter and complete the 1g7/2 proton orbital. The abundances of the cerium isotopes may differ very slightly in natural sources, because 138Ce and 140Ce are the daughters of the long-lived primordial radionuclides 138La and 144Nd, respectively.
Compounds
Cerium exists in two main oxidation states, Ce(III) and Ce(IV). This pair of adjacent oxidation states dominates several aspects of the chemistry of this element. Cerium(IV) aqueous solutions may be prepared by reacting cerium(III) solutions with the strong oxidizing agents peroxodisulfate orbismuthate Bismuthate is an ion. Its chemical formula is BiO3−. It has bismuth in its +5 oxidation state.
It is a very strong oxidizing agent. It reacts with hot water to make bismuth(III) oxide and oxygen. It also reacts with acids. Sodium bismuthate is t ...
. The value of ''E''⦵(Ce4+/Ce3+) varies widely depending on conditions due to the relative ease of complexation and hydrolysis with various anions, although +1.72 V is representative. Cerium is the only lanthanide which has important aqueous and coordination chemistry in the +4 oxidation state.
Halides
Cerium forms all four trihaldies CeX3 (X = F, Cl, Br, I) usually by reaction of the oxides with the hydrogen halides. The anhydrous halides are pale-colored, paramagnetic, hygroscopic solids. Upon hydration, the trihalides convert to complexes containing aquo complexes e(H2O)8-9sup>3+. Unlike most lanthanides, Ce forms a tetrafluoride, a white solid. It also forms a bronze-colored diiodide, which has metallic properties.Greenwood and Earnshaw, pp. 1240–2 Aside from the binary halide phases, a number of anionic halide complexes are known. Fluoride gives the Ce(IV) derivatives and . Chloride gives the orange .Oxides and chalcogenides
Cerium(IV) oxide ("ceria") has the fluorite structure, similarly to the dioxides of praseodymium and terbium. Ceria is anonstoichiometric compound
In chemistry, non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an empirical formula); mos ...
, meaning that the real formula is CeO2−x, where x is about 0.2. Thus, the material is not perfectly described as Ce(IV). Ceria reduces to cerium(III) oxide
Cerium(III) oxide, also known as cerium oxide, cerium trioxide, cerium sesquioxide, cerous oxide or dicerium trioxide, is an oxide of the rare-earth metal cerium. It has chemical formula and is gold-yellow in color.
Applications Engine and exha ...
with hydrogen gas.
Many nonstoichiometric
In chemistry, non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an empirical formula); mos ...
chalcogenides are also known, along with the trivalent Ce2Z3 (Z = S, Se, Te). The monochalcogenides CeZ conduct electricity and would better be formulated as Ce3+Z2−e−. While CeZ2 are known, they are polychalcogenides with cerium(III): cerium(IV) derivatives of S, Se, and Te are unknown.Greenwood and Earnshaw, pp. 1238–9
Cerium(IV) complexes
 The compound ceric ammonium nitrate ("CAN") is the most common cerium compound encountered in the laboratory. The six nitrate ligands bind as
The compound ceric ammonium nitrate ("CAN") is the most common cerium compound encountered in the laboratory. The six nitrate ligands bind as bidentate ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
s. The complex is 12-coordinate, a high coordination number which emphasizes the large size of the Ce4+ ion. CAN is popular oxidant in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
, both as a stoichiometric reagent and as a catalyst. It is inexpensive, easily handled. It operates by one-electron redox. Cerium nitrates also form 4:3 and 1:1 complexes with 18-crown-6
18-Crown-6 is an organic compound with the formula 2H4O and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point. Like other crown ethers, 18-crown-6 functions as a li ...
(the ratio referring to that between cerium and the crown ether).
Classically CAN] is a primary standard for quantitative analysis. Cerium(IV) salts, especially cerium(IV) sulfate
Cerium(IV) sulfate, also called ceric sulfate, is an inorganic compound. It exists as the anhydrous salt Ce( SO4)2 as well as a few hydrated forms: Ce(SO4)2(H2O)x, with x equal to 4, 8, or 12. These salts are yellow to yellow/orange solids that ...
, are often used as standard reagents for volumetric analysis
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to be analyzed). A reagent, termed the ''titrant'' ...
in cerimetric titrations.

 Due to ligand-to-metal charge transfer, aqueous cerium(IV) ions are orange-yellow. Aqueous cerium(IV) is metastable in water and is a strong oxidizing agent that oxidizes
Due to ligand-to-metal charge transfer, aqueous cerium(IV) ions are orange-yellow. Aqueous cerium(IV) is metastable in water and is a strong oxidizing agent that oxidizes hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
to give chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
gas.
In the Belousov–Zhabotinsky reaction, cerium oscillates between the +4 and +3 oxidation states to catalyze the reaction.
Organocerium compounds
Organocerium chemistry
Organocerium chemistry is the science exploring the properties, structure, and reactivity of organocerium compounds, chemical compounds that contain one or more chemical bond between carbon and cerium. These compounds comprise a subset of the orga ...
is similar to that of the other lanthanides, often involving complexes of cyclopentadienyl Cyclopentadienyl can refer to
*Cyclopentadienyl anion, or cyclopentadienide,
**Cyclopentadienyl ligand
*Cyclopentadienyl radical, •
*Cyclopentadienyl cation,
See also
*Pentadienyl
In organic chemistry, pentadienyl refers to the organic radic ...
and cyclooctatetraenyl
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as nnulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of ...
ligands. Cerocene
A lanthanocene is a type of metallocene compound that contains an Chemical element, element from the lanthanide series. The most common lanthanocene complexes contain two Sodium cyclopentadienide, cyclopentadienyl anions and an Covalent bond classi ...
(Ce(C8H8)2) adopts the uranocene molecular structure.Greenwood and Earnshaw, pp. 1248–9 The 4f electron in cerocene, , is poised ambiguously between being localized and delocalized and this compound is considered intermediate-valent.
Alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
, alkynyl, and alkenyl
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
organocerium derivatives are prepared from the transmetallation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
of the respective organolithium or Grignard reagents, and are more nucleophilic but less basic than their precursors.
History

 Cerium was discovered in
Cerium was discovered in Bastnäs
Bastnäs ( sv, Bastnäs or ) is an ore field near Riddarhyttan, Västmanland, Sweden. The mines in Bastnäs were earliest mentioned in 1692. Iron, copper and rare-earth elements were extracted from the mines and 4,500 tons of cerium was produced be ...
in Sweden by Jöns Jakob Berzelius
Jöns is a Swedish given name and a surname.
Notable people with the given name include:
* Jöns Jacob Berzelius (1779–1848), Swedish chemist
* Jöns Budde (1435–1495), Franciscan friar from the Brigittine monastery in NaantaliVallis Gratiae ...
and Wilhelm Hisinger, and independently in Germany by Martin Heinrich Klaproth, both in 1803. Cerium was named by Berzelius after the asteroid Ceres
Ceres most commonly refers to:
* Ceres (dwarf planet), the largest asteroid
* Ceres (mythology), the Roman goddess of agriculture
Ceres may also refer to:
Places
Brazil
* Ceres, Goiás, Brazil
* Ceres Microregion, in north-central Goiás st ...
, discovered two years earlier. The asteroid is itself named after the Roman goddess Ceres
Ceres most commonly refers to:
* Ceres (dwarf planet), the largest asteroid
* Ceres (mythology), the Roman goddess of agriculture
Ceres may also refer to:
Places
Brazil
* Ceres, Goiás, Brazil
* Ceres Microregion, in north-central Goiás st ...
, goddess of agriculture, grain crops, fertility and motherly relationships.
Cerium was originally isolated in the form of its oxide, which was named ''ceria'', a term that is still used. The metal itself was too electropositive to be isolated by then-current smelting technology, a characteristic of rare-earth metals in general. After the development of electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
by Humphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for t ...
five years later, the earths soon yielded the metals they contained. Ceria, as isolated in 1803, contained all of the lanthanides present in the cerite ore from Bastnäs, Sweden, and thus only contained about 45% of what is now known to be pure ceria. It was not until Carl Gustaf Mosander succeeded in removing lanthana and "didymia" in the late 1830s that ceria was obtained pure. Wilhelm Hisinger was a wealthy mine-owner and amateur scientist, and sponsor of Berzelius. He owned and controlled the mine at Bastnäs, and had been trying for years to find out the composition of the abundant heavy gangue rock (the "Tungsten of Bastnäs", which despite its name contained no tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolat ...
), now known as cerite, that he had in his mine. Mosander and his family lived for many years in the same house as Berzelius, and Mosander was undoubtedly persuaded by Berzelius to investigate ceria further.
The element played a role in the Manhattan Project
The Manhattan Project was a research and development undertaking during World War II that produced the first nuclear weapons. It was led by the United States with the support of the United Kingdom and Canada. From 1942 to 1946, the project w ...
, where cerium compounds were investigated in the Berkeley
Berkeley most often refers to:
*Berkeley, California, a city in the United States
**University of California, Berkeley, a public university in Berkeley, California
* George Berkeley (1685–1753), Anglo-Irish philosopher
Berkeley may also refer ...
site as materials for crucibles for uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
and plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibi ...
casting. For this reason, new methods for the preparation and casting of cerium were developed within the scope of the Ames
Ames may refer to:
Places United States
* Ames, Arkansas, a place in Arkansas
* Ames, Colorado
* Ames, Illinois
* Ames, Indiana
* Ames, Iowa, the most populous city bearing this name
* Ames, Kansas
* Ames, Nebraska
* Ames, New York
* Ames, Oklah ...
daughter project (now the Ames Laboratory). Production of extremely pure cerium in Ames commenced in mid-1944 and continued until August 1945.
Occurrence and production
Cerium is the most abundant of all the lanthanides, making up 66 ppm of the Earth's crust; this value is just behind that ofcopper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
(68 ppm), and cerium is even more abundant than common metals such as lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
(13 ppm) and tin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
(2.1 ppm). Thus, despite its position as one of the so-called rare-earth metal
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silve ...
s, cerium is actually not rare at all.Greenwood and Earnshaw, p. 1294 Cerium content in the soil varies between 2 and 150 ppm, with an average of 50 ppm; seawater contains 1.5 parts per trillion of cerium. Cerium occurs in various minerals, but the most important commercial sources are the minerals of the monazite and bastnäsite groups, where it makes up about half of the lanthanide content. Monazite-(Ce) is the most common representative of the monazites, with "-Ce" being the Levinson suffix informing on the dominance of the particular REE element representative. Also the cerium-dominant bastnäsite-(Ce) is the most important of the bastnäsites. Cerium is the easiest lanthanide to extract from its minerals because it is the only one that can reach a stable +4 oxidation state in aqueous solution.Greenwood and Earnshaw, pp. 1229–1232 Because of the decreased solubility of cerium in the +4 oxidation state, cerium is sometimes depleted from rocks relative to the other rare-earth elements and is incorporated into zircon
Zircon () is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is Zr SiO4. An empirical formula showing some of the r ...
, since Ce4+ and Zr4+ have the same charge and similar ionic radii. In extreme cases, cerium(IV) can form its own minerals separated from the other rare-earth elements, such as cerianite
Cerianite-(Ce) is a relatively rare oxide mineral, belonging to uraninite group with the formula . It is one of a few currently known minerals containing essential tetravalent cerium, the other examples being stetindite and dyrnaesite-(La).
Occur ...
(correctly named cerianite-(Ce)).
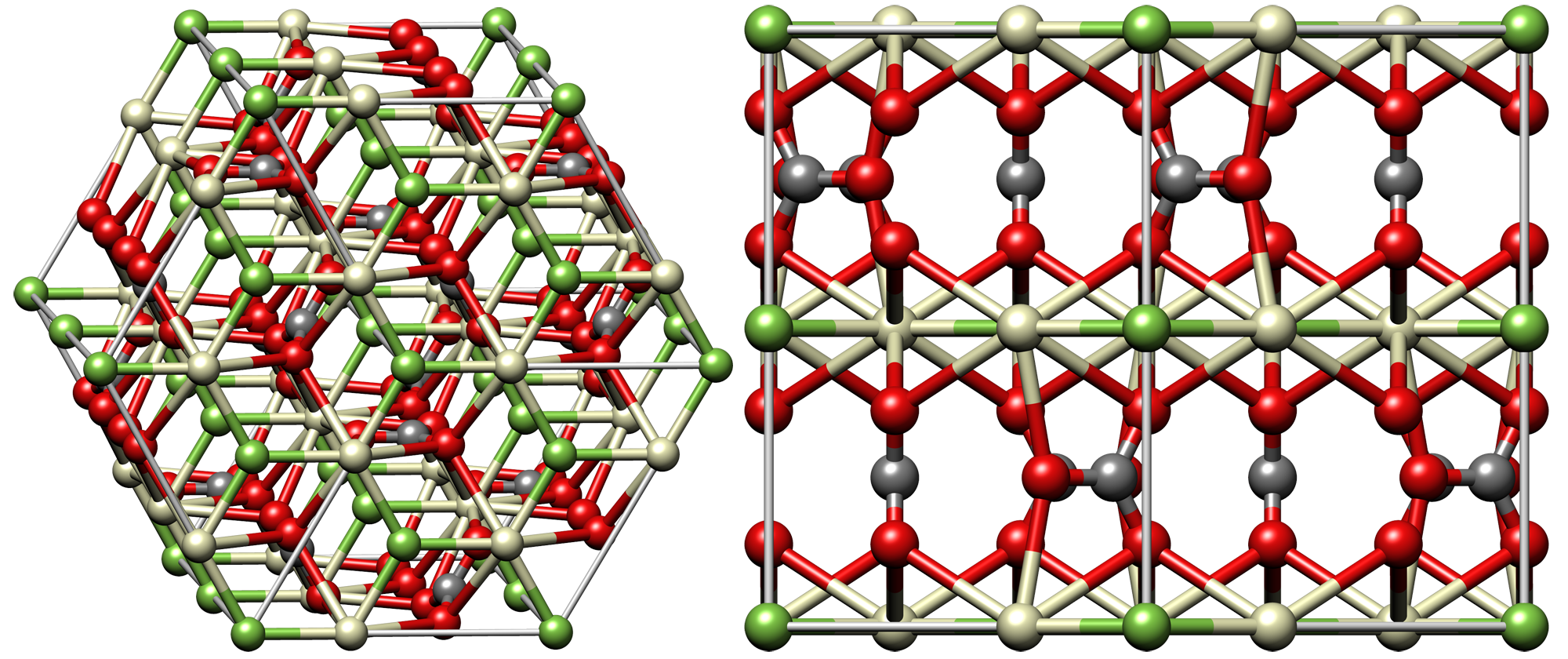
 Bastnäsite, LnIIICO3F, is usually lacking in
Bastnäsite, LnIIICO3F, is usually lacking in thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high me ...
and the heavy lanthanides beyond samarium and europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lanth ...
, and hence the extraction of cerium from it is quite direct. First, the bastnäsite is purified, using dilute hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
to remove calcium carbonate impurities. The ore is then roasted in the air to oxidize it to the lanthanide oxides: while most of the lanthanides will be oxidized to the sesquioxides Ln2O3, cerium will be oxidized to the dioxide CeO2. This is insoluble in water and can be leached out with 0.5 M hydrochloric acid, leaving the other lanthanides behind.
The procedure for monazite, , which usually contains all the rare earths, as well as thorium, is more involved. Monazite, because of its magnetic properties, can be separated by repeated electromagnetic separation. After separation, it is treated with hot concentrated sulfuric acid to produce water-soluble sulfates of rare earths. The acidic filtrates are partially neutralized with sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
to pH 3–4. Thorium precipitates out of solution as hydroxide and is removed. After that, the solution is treated with ammonium oxalate
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary amm ...
to convert rare earths to their insoluble oxalates. The oxalates are converted to oxides by annealing. The oxides are dissolved in nitric acid, but cerium oxide is insoluble in HNO3 and hence precipitates out. Care must be taken when handling some of the residues as they contain 228Ra, the daughter of 232Th, which is a strong gamma emitter.
Applications
 Cerium has two main applications, both of which use CeO2. The industrial application of ceria is for polishing, especially
Cerium has two main applications, both of which use CeO2. The industrial application of ceria is for polishing, especially chemical-mechanical planarization
Chemical mechanical polishing (CMP) or planarization is a process of smoothing surfaces with the combination of chemical and mechanical forces. It can be thought of as a hybrid of chemical etching and free abrasive polishing.
Description
The proc ...
(CMP). In its other main application, CeO2 is used to decolorize glass. It functions by converting green-tinted ferrous impurities to nearly colorless ferric oxides.
Sensors
Other automotive applications for the lower sesquioxide are as acatalytic converter
A catalytic converter is an exhaust emission control device that converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less-toxic pollutants by catalyzing a redox reaction. Catalytic converters are usually ...
for the oxidation of CO and NO''x'' emissions in the exhaust gases from motor vehicles, Ceria has also been used as a substitute for its radioactive congener thoria
''Thoria'' is a genus of shield bugs in the tribe Podopini
Podopinae, known as turtle bugs, are a subfamily of the insect family Pentatomidae. The type genus is '' Podops''.
Tribes and Genera
''BioLib'' lists: Brachycerocorini
Auth. Davidova ...
, for example in the manufacture of electrodes used in gas tungsten arc welding, where ceria as an alloying element improves arc stability and ease of starting while decreasing burn-off.
Gas mantles and pyrophoric alloys
The first use of cerium was in gas mantles, invented by Austrian chemist Carl Auer von Welsbach. In 1885, he had previously experimented with mixtures ofmagnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
, lanthanum, and yttrium oxides, but these gave green-tinted light and were unsuccessful. Six years later, he discovered that pure thorium oxide produced a much better, though blue, light, and that mixing it with cerium dioxide resulted in a bright white light. Cerium dioxide also acts as a catalyst for the combustion of thorium oxide.
This resulted in commercial success for von Welsbach and his invention, and created great demand for thorium. Its production resulted in a large amount of lanthanides being simultaneously extracted as by-products.Greenwood and Earnshaw, p. 1228 Applications were soon found for them, especially in the pyrophoric alloy known as " mischmetal" composed of 50% cerium, 25% lanthanum, and the remainder being the other lanthanides, that is used widely for lighter flints. Usually iron is added to form the alloy ferrocerium
Ferrocerium (also known in Europe as Auermetall) is a synthetic pyrophoric alloy of mischmetal (cerium, lanthanum, neodymium, other trace lanthanides and some iron – about 95% lanthanides and 5% iron) hardened by blending in oxides of ...
, also invented by von Welsbach. Due to the chemical similarities of the lanthanides, chemical separation is not usually required for their applications, such as the addition of mischmetal to steel as an inclusion modifier to improve mechanical properties, or as catalysts for the cracking of petroleum. This property of cerium saved the life of writer Primo Levi
Primo Michele Levi (; 31 July 1919 – 11 April 1987) was an Italian chemist, partisan, writer, and Jewish Holocaust survivor. He was the author of several books, collections of short stories, essays, poems and one novel. His best-known works ...
at the Auschwitz concentration camp
Auschwitz concentration camp ( (); also or ) was a complex of over 40 concentration and extermination camps operated by Nazi Germany in occupied Poland (in a portion annexed into Germany in 1939) during World War II and the Holocaust. It con ...
, when he found a supply of ferrocerium alloy and bartered it for food.
Pigments and phosphors
The photostability ofpigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compo ...
s can be enhanced by the addition of cerium, as it provides pigments with lightfastness
Lightfastness is a property of a colourant such as dye or pigment that describes its resistance to fading when exposed to light. Dyes and pigments are used for example for dyeing of fabrics, plastics or other materials and manufacturing paints or ...
and prevents clear polymers from darkening in sunlight.
An example of a cerium compound used on its own as an inorganic pigment is the vivid red cerium(III) sulfide
Cerium(III) sulfide, also known as cerium sesquisulfide, is an inorganic compound with the formula Ce2S3. It is the sulfide salt of Cerium#Chemistry, cerium(III) and exists as three Polymorphism (materials science), polymorphs with different cryst ...
(cerium sulfide red), which stays chemically inert up to very high temperatures. The pigment is a safer alternative to lightfast but toxic cadmium selenide-based pigments.
The addition of cerium oxide to older cathode-ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms (oscilloscope), pictur ...
television glass plates was beneficial, as it suppresses the darkening effect from the creation of F-center defects due to the continuous electron bombardment during operation.
Cerium is also an essential component as a dopant
A dopant, also called a doping agent, is a trace of impurity element that is introduced into a chemical material to alter its original electrical or optical properties. The amount of dopant necessary to cause changes is typically very low. When ...
for phosphor
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or vi ...
s used in CRT TV screens, fluorescent lamps, and later white light-emitting diodes.Cerium dioxide. nanopartikel.info (2011-02-02) The most commonly used example is cerium(III)-doped yttrium aluminium garnet (Ce:YAG) which emits green to yellow-green light (550–530 nm) and also behaves as a
scintillator
A scintillator is a material that exhibits scintillation, the property of luminescence, when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate (i.e. re-emit the absorbed ...
.
Other alloys and refractories
Cerium salts, such as the sulfides Ce2S3 and Ce3S4, were considered during theManhattan Project
The Manhattan Project was a research and development undertaking during World War II that produced the first nuclear weapons. It was led by the United States with the support of the United Kingdom and Canada. From 1942 to 1946, the project w ...
as advanced refractory materials
In materials science, a refractory material or refractory is a material that is resistant to Thermal decomposition, decomposition by heat, pressure, or chemical attack, and retains strength and form at high temperatures. Refractories are polycr ...
for the construction of crucibles which could withstand the high temperatures and strongly reducing conditions when casting plutonium metal. Despite desirable properties, these sulfides were never widely adopted due to practical issues with their synthesis.
Cerium is used as alloying element in aluminum to create castable eutectic aluminum alloys
An aluminium alloy (or aluminum alloy; see spelling differences) is an alloy in which aluminium (Al) is the predominant metal. The typical alloying elements are copper, magnesium, manganese, silicon, tin, nickel and zinc. There are two principa ...
with 6–16 wt.% Ce, to which Mg and/or Si can be further added. These Al-Ce alloys have excellent high temperature strength and are suitable for automotive applications ''e.g.'' in cylinder head
In an internal combustion engine, the cylinder head (often abbreviated to simply "head") sits above the cylinders and forms the roof of the combustion chamber.
In sidevalve engines, the head is a simple sheet of metal; whereas in more modern ov ...
s. Other alloys of cerium include Pu-Ce and Pu-Ce-Co plutonium alloys, which have been used as nuclear fuel
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergoing ...
.
Biological role and precautions
The early lanthanides have been found to be essential to somemethanotrophic
Methanotrophs (sometimes called methanophiles) are prokaryotes that metabolize methane as their source of carbon and chemical energy. They are bacteria or archaea, can grow aerobically or anaerobically, and require single-carbon compounds to s ...
bacteria living in volcanic mudpots, such as ''Methylacidiphilum fumariolicum
''Methylacidiphilum fumariolicum '' is an autotrophic bacterium first described in 2007 growing on volcanic pools near Naples, Italy. It grows in mud at temperatures between 50 °C and 60 °C and an acidic pH of 2–5. It is able to oxi ...
'': lanthanum, cerium, praseodymium, and neodymium are about equally effective. Cerium is otherwise not known to have biological role in any other organisms, but is not very toxic either; it does not accumulate in the food chain to any appreciable extent. Because it often occurs together with calcium in phosphate minerals, and bones are primarily calcium phosphate, cerium can accumulate in bones in small amounts that are not considered dangerous. Cerium, like the other lanthanides, is known to affect human metabolism, lowering cholesterol levels, blood pressure, appetite, and risk of blood coagulation.
Cerium nitrate is an effective topical antimicrobial treatment for third-degree burn
A burn is an injury to skin, or other tissues, caused by heat, cold, electricity, chemicals, friction, or ultraviolet radiation (like sunburn). Most burns are due to heat from hot liquids (called scalding), solids, or fire. Burns occur mainl ...
s, although large doses can lead to cerium poisoning and methemoglobinemia
Methemoglobinemia, or methaemoglobinaemia, is a condition of elevated methemoglobin in the blood. Symptoms may include headache, dizziness, shortness of breath, nausea, poor muscle coordination, and blue-colored skin (cyanosis). Complications m ...
. The early lanthanides act as essential cofactors for the methanol dehydrogenase of the methanotrophic
Methanotrophs (sometimes called methanophiles) are prokaryotes that metabolize methane as their source of carbon and chemical energy. They are bacteria or archaea, can grow aerobically or anaerobically, and require single-carbon compounds to s ...
bacterium ''Methylacidiphilum fumariolicum
''Methylacidiphilum fumariolicum '' is an autotrophic bacterium first described in 2007 growing on volcanic pools near Naples, Italy. It grows in mud at temperatures between 50 °C and 60 °C and an acidic pH of 2–5. It is able to oxi ...
'' SolV, for which lanthanum, cerium, praseodymium, and neodymium alone are about equally effective.
Like all rare-earth metals, cerium is of low to moderate toxicity. A strong reducing agent, it ignites spontaneously in air at 65 to 80 °C. Fumes from cerium fires are toxic. Water should not be used to stop cerium fires, as cerium reacts with water to produce hydrogen gas. Workers exposed to cerium have experienced itching, sensitivity to heat, and skin lesions. Cerium is not toxic when eaten, but animals injected with large doses of cerium have died due to cardiovascular collapse. Cerium is more dangerous to aquatic organisms, on account of being damaging to cell membranes; this is an important risk because it is not very soluble in water, thus causing contamination of the environment .
References
Bibliography
* {{Authority control Chemical elements Chemical elements with double hexagonal close-packed structure Lanthanides Reducing agents