|
Photodisintegration
Photodisintegration (also called phototransmutation, or a photonuclear reaction) is a nuclear process in which an atomic nucleus absorbs a high-energy gamma ray, enters an excited state, and immediately decays by emitting a subatomic particle. The incoming gamma ray effectively knocks one or more neutrons, protons, or an alpha particle out of the nucleus. The reactions are called (γ,n), (γ,p), and (γ,α), respectively. Photodisintegration is endothermic (energy absorbing) for atomic nuclei lighter than iron and sometimes exothermic (energy releasing) for atomic nuclei heavier than iron. Photodisintegration is responsible for the nucleosynthesis of at least some heavy, proton-rich elements via the p-process in supernovae of type Ib, Ic, or II. This causes the iron to further fuse into the heavier elements. Photodisintegration of deuterium A photon carrying 2.22 MeV or more energy can photodisintegrate an atom of deuterium: : James Chadwick and Maurice Goldhaber used this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supernova
A supernova (: supernovae or supernovas) is a powerful and luminous explosion of a star. A supernova occurs during the last stellar evolution, evolutionary stages of a massive star, or when a white dwarf is triggered into runaway nuclear fusion. The original object, called the ''progenitor'', either collapses to a neutron star or black hole, or is completely destroyed to form a diffuse nebula. The peak optical luminosity of a supernova can be comparable to that of an entire galaxy before fading over several weeks or months. The last supernova directly observed in the Milky Way was Kepler's Supernova in 1604, appearing not long after Tycho's Supernova in 1572, both of which were visible to the naked eye. The supernova remnant, remnants of more recent supernovae have been found, and observations of supernovae in other galaxies suggest they occur in the Milky Way on average about three times every century. A supernova in the Milky Way would almost certainly be observable through mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P-process
The term p-process (''p'' for proton) is used in two ways in the scientific literature concerning the astrophysical origin of the elements (nucleosynthesis). Originally it referred to a proton capture process which was proposed to be the source of certain, naturally occurring, neutron-deficient isotopes of the elements from selenium to mercury. These nuclides are called p-nuclei and their origin is still not completely understood. Although it was shown that the originally suggested process cannot produce the p-nuclei, later on the term p-process was sometimes used to generally refer to any nucleosynthesis process supposed to be responsible for the p-nuclei. Often, the two meanings are confused. Recent scientific literature therefore suggests to use the term p-process only for the actual proton capture process, as it is customary with other nucleosynthesis processes in astrophysics. The proton capture p-process Proton-rich nuclides can be produced by sequentially adding one or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
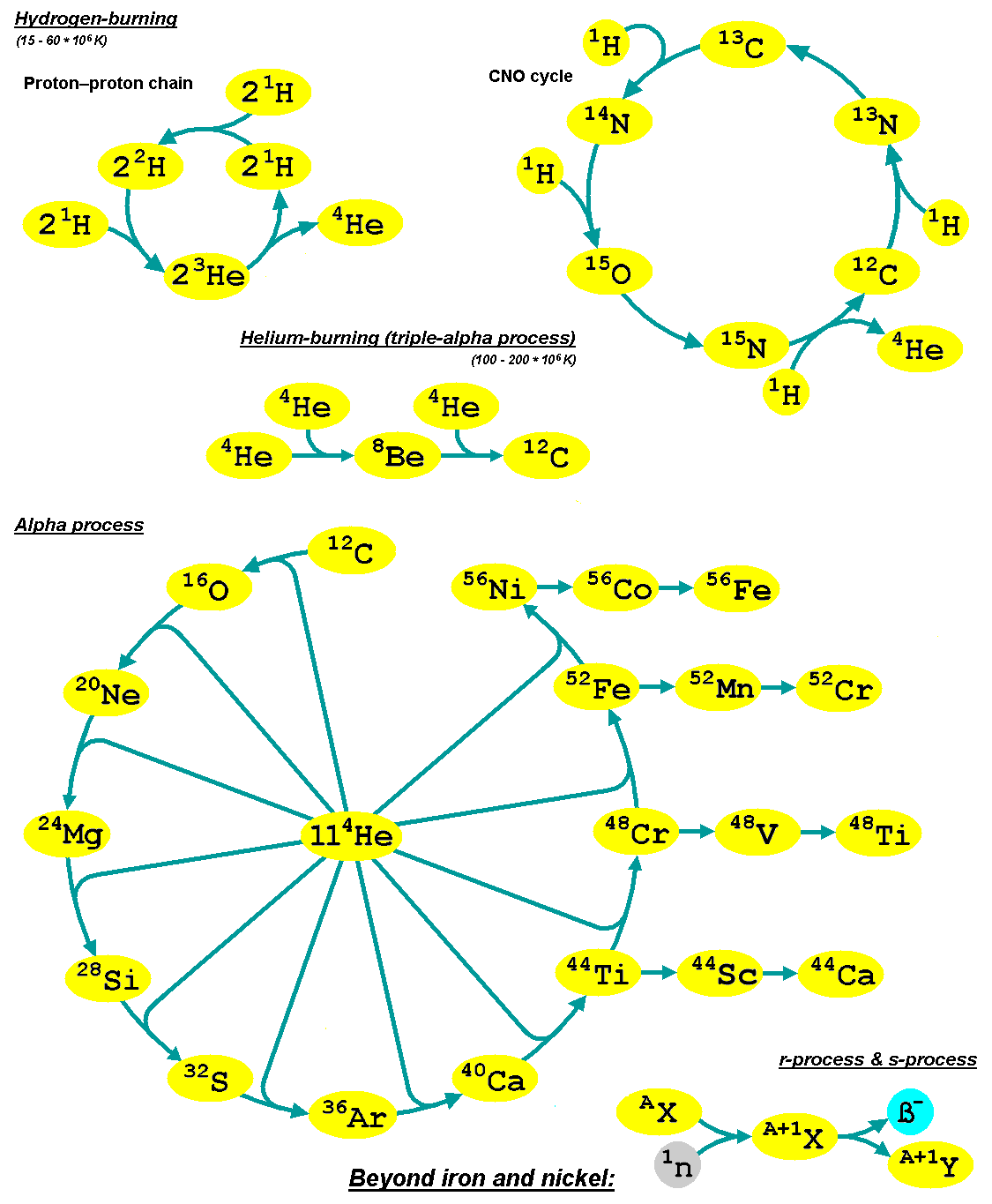
Nucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in a process called Big Bang nucleosynthesis. After about 20 minutes, the universe had expanded and cooled to a point at which these high-energy collisions among nucleons ended, so only the fastest and simplest reactions occurred, leaving our universe containing hydrogen and helium. The rest is traces of other elements such as lithium and the hydrogen isotope deuterium. Nucleosynthesis in stars and their explosions later produced the variety of elements and isotopes that we have today, in a process called cosmic chemical evolution. The amounts of total mass in elements heavier than hydrogen and helium (called 'metals' by astrophysicists) remains small (few percent), so that the universe still has approximately the same composition. Stars ste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most abundant element in the Earth's crust, being mainly deposited by meteorites in its metallic state. Extracting usable metal from iron ores requires kilns or furnaces capable of reaching , about 500 °C (900 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BC and the use of iron tools and weapons began to displace copper alloys – in some regions, only around 1200 BC. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, due to their mechan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Source
A neutron source is any device that emits neutrons, irrespective of the mechanism used to produce the neutrons. Neutron sources are used in physics, engineering, medicine, nuclear weapons, petroleum exploration, biology, chemistry, and nuclear power. Neutron source variables include the energy of the neutrons emitted by the source, the rate of neutrons emitted by the source, the size of the source, the cost of owning and maintaining the source, and government regulations related to the source. Small devices Spontaneous fission Some isotopes undergo spontaneous fission (SF) with Neutron emission, emission of neutrons. The most common spontaneous fission source is the isotope californium-252. 252Cf and all other SF neutron sources are made by irradiating uranium or a Transuranium element, transuranic element in a nuclear reactor, where neutrons are absorbed in the starting material and its subsequent reaction products, transmuting the starting material into the SF isotope. 252Cf n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma Ray
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz () and wavelengths less than 10 picometers (), gamma ray photons have the highest photon energy of any form of electromagnetic radiation. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation ''gamma rays'' based on their relatively strong penetration of matter; in 1900, he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power. Gamma rays from radioactive decay are in the energy range ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Startup Neutron Source
A startup neutron source is a neutron source used for stable and reliable initiation of nuclear chain reaction in nuclear reactors, when they are loaded with fresh nuclear fuel, whose neutron flux from spontaneous fission is insufficient for a reliable startup, or after prolonged shutdown periods. Neutron sources ensure a constant minimal population of neutrons in the reactor core, sufficient for a smooth startup. Without them, the reactor could suffer fast power excursions during startup from state with too few self-generated neutrons (new core or after extended shutdown). The startup sources are typically inserted in regularly spaced positions inside the reactor core, in place of some of the fuel rods. The sources are important for safe reactor startup. The spontaneous fission and ambient radiation such as cosmic rays serve as weak neutron sources, but these are too weak for the reactor instrumentation to detect; relying on them could lead to a "blind" start, which is a pote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Temperature
The neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term ''temperature'' is used, since hot, thermal and cold neutrons are moderated in a medium with a certain temperature. The neutron energy distribution is then adapted to the Maxwell distribution known for thermal motion. Qualitatively, the higher the temperature, the higher the kinetic energy of the free neutrons. The momentum and wavelength of the neutron are related through the de Broglie relation. The long wavelength of slow neutrons allows for the large cross section. Neutron energy distribution ranges The precise boundaries of neutron energy ranges are not well defined, and differ between sources, but some common names and limits are given in the following table. The following is a detailed classification: Thermal A thermal neutron is a free neutron with a kinetic energy of about 0.025 eV (about 4.0×10−21 J or 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-12
Carbon-12 (12C) is the most abundant of the two stable isotopes of carbon ( carbon-13 being the other), amounting to 98.93% of element carbon on Earth; its abundance is due to the triple-alpha process by which it is created in stars. Carbon-12 is of particular importance in its use as the standard from which atomic masses of all nuclides are measured, thus, its atomic mass is exactly 12 daltons by definition. Carbon-12 is composed of 6 protons, 6 neutrons, and 6 electrons. History Before 1959, both the IUPAP and IUPAC used oxygen to define the mole; the chemists defining the mole as the number of atoms of oxygen which had mass 16 g, the physicists using a similar definition but with the oxygen-16 isotope only. The two organizations agreed in 1959–60 to define the mole as follows. ''Mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 12 gram of carbon 12; its symbol is "mol".'' This was adopted by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Mass
The solar mass () is a frequently used unit of mass in astronomy, equal to approximately . It is approximately equal to the mass of the Sun. It is often used to indicate the masses of other stars, as well as stellar clusters, nebulae, galaxies and black holes. More precisely, the mass of the Sun is The solar mass is about times the mass of Earth (), or times the mass of Jupiter (). History of measurement The value of the gravitational constant was first derived from measurements that were made by Henry Cavendish in 1798 with a torsion balance. The value he obtained differs by only 1% from the modern value, but was not as precise. The diurnal parallax of the Sun was accurately measured during the transits of Venus in 1761 and 1769, yielding a value of (9 arcseconds, compared to the present value of ). From the value of the diurnal parallax, one can determine the distance to the Sun from the geometry of Earth. The first known estimate of the solar mass was by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Process
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a transformation of at least one nuclide to another. If a nucleus interacts with another nucleus or particle, they then separate without changing the nature of any nuclide, the process is simply referred to as a type of nuclear scattering, rather than a nuclear reaction. In principle, a reaction can involve more than two particles colliding, but because the probability of three or more nuclei to meet at the same time at the same place is much less than for two nuclei, such an event is exceptionally rare (see triple alpha process for an example very close to a three-body nuclear reaction). The term "nuclear reaction" may refer either to a change in a nuclide induced by collision with another particle or to a spontaneous change of a nuclide without collisi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony-124
Antimony (Sb) occurs in two stable isotopes, Sb and Sb. There are 37 artificial radioactive isotopes, the longest-lived of which are Sb, with a half-life of 2.75856 years; Sb, with half-life 60.2 days; and Sb, with half-life 12.35 days. All other isotopes have half-lives less than 4 days, most less than an hour. There are also many isomers, the longest-lived of which is Sb with half-life 5.76 days. List of isotopes , -id=Antimony-104 , rowspan=4, 104Sb , rowspan=4 style="text-align:right" , 51 , rowspan=4 style="text-align:right" , 53 , rowspan=4, 103.93634(11)# , rowspan=4, 470(130) ms , β+? , 104Sn , rowspan=4, , rowspan=4, , rowspan=4, , - , p (<7%) , 103Sn , - , β+, p (<7%) , 103In , - , α? , 100In , -id=Antimony-105 , rowspan=3, 105Sb , rowspan=3 style="text-align:right" , 51 , rowspan=3 style= ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |