Buchwald–Hartwig Amination on:
[Wikipedia]
[Google]
[Amazon]
In 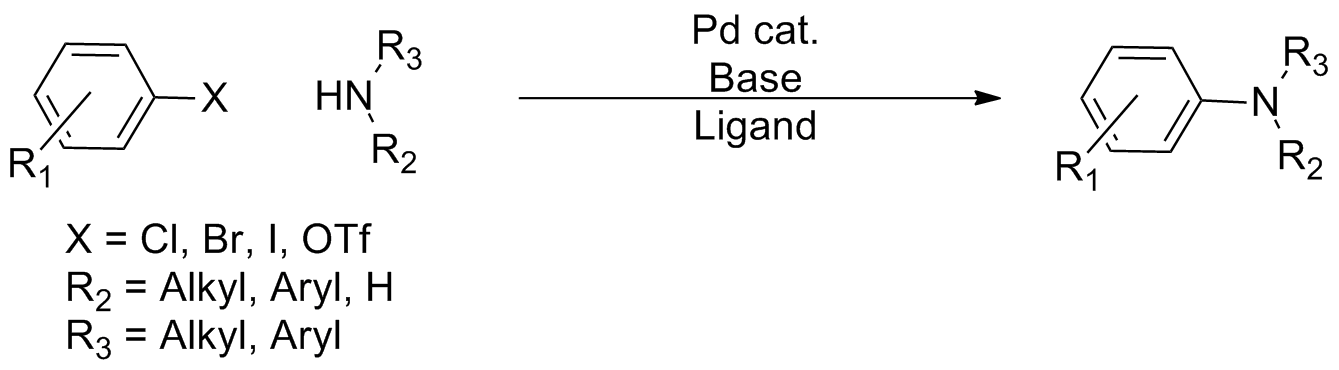 Over the course of its development, several 'generations' of catalyst systems have been developed, with each system allowing greater scope in terms of coupling partners and milder conditions, allowing virtually any amine to be coupled with a wide variety of aryl coupling partners. Because of the ubiquity of aryl C-N bonds in pharmaceuticals and
Over the course of its development, several 'generations' of catalyst systems have been developed, with each system allowing greater scope in terms of coupling partners and milder conditions, allowing virtually any amine to be coupled with a wide variety of aryl coupling partners. Because of the ubiquity of aryl C-N bonds in pharmaceuticals and

Buchwald–Hartwig Coupling – Recent Literature
* ''Buchwald–Hartwig Chemistry'' Ian Mangion MacMillan Group Meeting July 30, 200
Link
* ''Buchwald–Hartwig reaction Precious-Metal catalysts from Acros Organics for coupling reactions in organic synthesis'
Link
{{DEFAULTSORT:Buchwald-Hartwig amination Substitution reactions Name reactions
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, the Buchwald–Hartwig amination is a chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
for the synthesis of carbon–nitrogen bond A carbon–nitrogen bond is a covalent bond between carbon and nitrogen and is one of the most abundant bonds in organic chemistry and biochemistry.
Nitrogen has five valence electrons and in simple amines it is trivalent, with the two remaining el ...
s via the palladium-catalyzed coupling reactions of amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s with aryl halides. Although Pd-catalyzed C-N couplings were reported as early as 1983, Stephen L. Buchwald and John F. Hartwig
John F. Hartwig is an American organometallic chemist who holds the position of Henry Rapoport Professor of Chemistry at the University of California, Berkeley. His laboratory traditionally focuses on developing transition metal-catalyzed reacti ...
have been credited, whose publications between 1994 and the late 2000s established the scope of the transformation. The reaction's synthetic utility stems primarily from the shortcomings of typical methods ( nucleophilic substitution, reductive amination, etc.) for the synthesis of aromatic bonds, with most methods suffering from limited substrate scope and functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
tolerance. The development of the Buchwald–Hartwig reaction allowed for the facile synthesis of aryl amines, replacing to an extent harsher methods (the Goldberg reaction Goldberg or Goldberger may refer to:
Arts and entertainment
* Goldberg Ensemble, a British string ensemble
* ''Goldberg Variations'', a set of 30 keyboard variations by Johann Sebastian Bach
* ''The Goldbergs (broadcast series)'', American radio ...
, nucleophilic aromatic substitution, etc.) while significantly expanding the repertoire of possible bond formation.
:  Over the course of its development, several 'generations' of catalyst systems have been developed, with each system allowing greater scope in terms of coupling partners and milder conditions, allowing virtually any amine to be coupled with a wide variety of aryl coupling partners. Because of the ubiquity of aryl C-N bonds in pharmaceuticals and
Over the course of its development, several 'generations' of catalyst systems have been developed, with each system allowing greater scope in terms of coupling partners and milder conditions, allowing virtually any amine to be coupled with a wide variety of aryl coupling partners. Because of the ubiquity of aryl C-N bonds in pharmaceuticals and natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical syn ...
s, the reaction has gained wide use in synthetic organic chemistry, with application in many total syntheses
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes ...
and the industrial preparation of numerous pharmaceuticals.
History
The first example of a palladium catalyzed C–N cross-coupling reaction was published in 1983 by Migita and coworkers and described a reaction between several aryl bromides and N,N-diethylamino-tributyltin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
using 1 mol% PdCl2 (o-tolyl)3sub>2. Though several aryl bromides were tested, only electronically neutral, sterically unencumbered substrates gave good to excellent yields.
In 1984, Dale L. Boger
Dale Lester Boger is an American medicinal and organic chemist and former chair of the Department of Chemistry at The Scripps Research Institute in La Jolla, CA.
Dale Boger was born on August 22, 1953, in Hutchinson, Kansas. He studied chemist ...
and James S. Panek reported an example of Pd(0)-mediated C–N bond formation in the context of their work on the synthesis of lavendamycin which utilized stoichiometric Pd(PPh3)4. Attempts to render the reaction catalytic were unsuccessful.
These reports were virtually uncited for a decade. In February 1994, Hartwig reported a systematic study of the palladium compounds involved in the original Migita paper, concluding that the d10 complex Pd (o-Tolyl)3sub>2 was the active catalyst. Proposed was a catalytic cycle involving oxidative addition of the aryl bromide.
In May 1994, Buchwald published an extension of the Migita paper offering two major improvements over the original paper. First, transamination of Bu3SnNEt2 followed by argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
purge to remove the volatile diethylamine allowed extension of the methodology to a variety of secondary amines (both cyclic and acyclic) and primary aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine
In organic chemistry, an aromatic amine is an organic compound consisting of an aroma ...
s. Secondly, the yield for electron rich and electron poor arenes was improved via minor modifications to the reaction procedure (higher catalyst loading, higher temperature, longer reaction time), although no ortho-substituted aryl groups were included in this publication.
In 1995, back to back studies from each lab showed that the couplings could be conducted with free amines in the presence of a bulky base ( NaOtBu in the Buchwald publication, LiHMDS
Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula . It is commonly abbreviated as LiHMDS or Li(HMDS) (lithium hexamethyldisilazide - a reference to its conjugate acid HMDS) and is primarily used as a strong ...
in the Hartwig publication), allowing for organotin
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide (), discovered by ...
-free coupling. Though these improved conditions proceeded at a faster rate, the substrate scope was limited almost entirely to secondary amines due to competitive hydrodehalogenation of the bromoarenes. (See Mechanism
Mechanism may refer to:
*Mechanism (engineering), rigid bodies connected by joints in order to accomplish a desired force and/or motion transmission
*Mechanism (biology), explaining how a feature is created
*Mechanism (philosophy), a theory that a ...
below)
These results established the so-called "first generation" of Buchwald–Hartwig catalyst systems. The following years saw development of more sophisticated phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
ligands
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electro ...
that allowed extension to a larger variety of amines and aryl groups. Aryl iodides, chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts ...
s, and triflates eventually became suitable substrates, and reactions run with weaker bases at room temperature were developed. These advances are detailed in the Scope
Scope or scopes may refer to:
People with the surname
* Jamie Scope (born 1986), English footballer
* John T. Scopes (1900–1970), central figure in the Scopes Trial regarding the teaching of evolution
Arts, media, and entertainment
* Cinem ...
section below, and the extension to more complex systems remains an active area of research.
Mechanism
Thereaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
for this reaction has been demonstrated to proceed through steps similar to those known for palladium catalyzed C-C coupling reactions. Steps include oxidative addition of the aryl halide to a Pd(0) species, addition of the amine to the oxidative addition complex, deprotonation followed by reductive elimination. An unproductive side reaction can compete with reductive elimination wherein the amide undergoes beta hydride elimination to yield the hydrodehalogenated arene and an imine product.
Throughout the development of the reaction the group sought to identify reaction intermediates through fundamental mechanistic studies. These studies have revealed a divergent reaction pathways depending on whether monodentate or chelating phosphine ligands are employed in the reaction, and a number of nuanced influences have been revealed (especially concerning the dialkylbiaryl phosphine ligands developed by Buchwald).
The catalytic cycle proceeds as follows:
For monodentate ligand systems the monophosphine palladium (0) species is believed to form the palladium (II) species which is in equilibrium with the μ-halogen dimer. The stability of this dimer decreases in the order of X = I > Br > Cl, and is thought to be responsible for the slow reaction of aryl iodides with the first-generation catalyst system. Amine ligation followed by deprotonation by base produces the palladium amide. (Chelating systems have been shown to undergo these two steps in reverse order, with base complexation preceding amide formation.) This key intermediate reductively eliminates to produce the product and regenerate the catalyst. However, a side reaction can occur wherein β-hydride elimination followed by reductive elimination produces the hydrodehalogenated arene and the corresponding imine. Not shown are additional equilibria wherein various intermediates coordinate to additional phosphine ligands at various stages in the catalytic cycle.
For chelating ligands, the monophosphine palladium species is not formed; oxidative addition, amide formation and reductive elimination occur from L2Pd complexes. The Hartwig group found that "reductive elimination can occur from either a four-coordinate bisphosphine or three-coordinate monophosphine arylpalladium amido complex. Eliminations from the three-coordinate compounds are faster. Second, β-hydrogen elimination occurs from a three-coordinate intermediate. Therefore, β-hydrogen elimination occurs slowly from arylpalladium complexes containing chelating phosphines while reductive elimination can still occur from these four-coordinate species."
Application
Because of the ubiquity of aryl C-N bonds in pharmaceuticals andnatural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical syn ...
s, the reaction has gained wide use in synthetic organic chemistry, with application in many total syntheses
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes ...
and the industrial preparation of numerous pharmaceuticals.
Industrial applications include α-arylation of carbonyl compounds (such as ketones, esters, amides, aldehydes) and nitriles.
Scope
Although the scope of the Buchwald–Hartwig amination has been expanded to include a wide variety of aryl and amine coupling partners, the conditions required for any particular reactants are still largely substrate dependent. Various ligand systems have been developed, each with varying capabilities and limitations, and the choice of conditions requires consideration of the steric and electronic properties of both partners. Detailed below are the substrates and conditions for the major generations of ligand systems. (Not included herein areN-heterocyclic carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for ex ...
ligands and ligands with wide bite angles such as Xantphos
Xantphos is an organophosphorus compound derived from the heterocycle xanthene. It is used as a bidentate diphosphine ligand and is noteworthy for having a particularly wide bite angle (108°). Such ligands are useful in the hydroformylation of ...
and Spanphos which also have been developed considerably.)
First-generation catalyst system
The first generation (Pd (o-Tolyl)3sub>2) catalyst system was found to be effective for the coupling of both cyclic and acyclic secondary amines bearing both alkyl and aryl functionality (though not diarylamines) with a variety of aryl bromides. In general, these conditions were not able to couple primary amines due to competitive hydrodehalogenation of the arene. Aryl iodides were found to be suitable substrates for the intramolecular variant of this reaction, and importantly, could be coupled intermolecularly only if dioxane was used in place of toluene as a solvent, albeit with modest yields.
Bidentate phosphine ligands
The development of diphenylphosphinobinapthyl (BINAP) and diphenylphosphinoferrocene (DPPF) as ligands for the Buchwald–Hartwig amination provided the first reliable extension to primary amines and allowed efficient coupling of aryl iodides and triflates. (It is believed that the bidentate ligands prevent formation of the palladium iodide dimer after oxidative addition, speeding up the reaction.) These ligands typically produce the coupled products at higher rates and better yields than the first generation of catalysts. The initial reports of these ligands as catalysts were somewhat unexpected given the mechanistic evidence for monoligated complexes serving as the active catalysts in the first-generation system. In fact, the first examples from both labs were published in the same issue of JACS. The chelation from these ligands is thought to suppress β-hydride elimination by preventing an open coordination site. In fact, α-chiral amines were found not to racemize when chelating ligands were employed, in contrast to the first-generation catalyst system.Sterically hindered ligands
Bulky tri- and di-alkyl phosphine ligands have been shown to be remarkably active catalysts, allowing the coupling of a wide range of amines (primary, secondary, electron withdrawn, heterocyclic, etc.) with aryl chlorides, bromides, iodides, and triflates. Additionally, reactions employinghydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
, carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate g ...
, and phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phospho ...
bases in place of the traditional alkoxide and silylamide bases have been developed. The Buchwald group has developed a wide range of dialkylbiaryl phosphine ligands, while the Hartwig group has focused on ferrocene
Ferrocene is an organometallic compound with the formula . The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, a ...
-derived and trialkyl phosphine ligands.
The dramatic increase in activity seen with these ligands is attributed to their propensity to sterically favor the monoligated palladium species at all stages of the catalytic cycle, dramatically increasing the rate of oxidative addition, amide formation, and reductive elimination. Several of these ligands also seem to enhance the rate of reductive elimination relative to β-hydride elimination via the electron donating arene-palladium interaction.
Even electron withdrawn amines and heterocyclic substrates can be coupled under these conditions, despite their tendency to deactivate the palladium catalyst.
Ammonia equivalents
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
remains one of the most challenging coupling partners for Buchwald–Hartwig amination reactions, a problem attributed to its tight binding with palladium complexes. Several strategies have been developed to overcome this based on reagents that serve as ammonia equivalents. The use of a benzophenone imine
Benzophenone imine is an organic compound with the formula of (C6H5)2C=NH. A pale yellow liquid, benzophenone imine is used as a reagent in organic synthesis.
Synthesis
Benzophenone imine can be prepared by the thermal decomposition of benzophen ...
or silylamide can overcome this limitation, with subsequent hydrolysis furnishing the primary aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine
In organic chemistry, an aromatic amine is an organic compound consisting of an aroma ...
.
A catalyst system that can directly couple ammonia using a Josiphos-type ligand.
Variations on C-N couplings: C-O, C-S, and C-C couplings
Under conditions similar to those employed for amination,alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s can be coupled with aryl halides to produce the corresponding aryl ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be c ...
s. This serves as a convenient replacement for harsher analogues of this process such as the Ullmann condensation
The Ullmann condensation or Ullmann-type reaction is the copper-promoted conversion of aryl halides to aryl ethers, aryl thioethers, aryl nitriles, and aryl amines. These reactions are examples of cross-coupling reactions.
Ullmann-type reactions ...
.
Thiols and thiophenols can be coupled with aryl halides under Buchwald-Hartwig-type conditions to produce the corresponding aryl thioethers. Furthermore, mercaptoesters has been employed as H2S-equivalents in order to generate the thiophenol from the corresponding aryl halide.
Enolates and other similar carbon nucleophiles can also be coupled to produce α-aryl ketones, malonates, nitriles, etc. The scope of this transformation is similarly ligand-dependent and a number of systems have been developed. Several enantioselective methods for this process have been developed.
Several versions of the reaction employing complexes of copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
and nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to ...
rather than palladium have also been developed.
References
External links
Buchwald–Hartwig Coupling – Recent Literature
* ''Buchwald–Hartwig Chemistry'' Ian Mangion MacMillan Group Meeting July 30, 200
Link
* ''Buchwald–Hartwig reaction Precious-Metal catalysts from Acros Organics for coupling reactions in organic synthesis'
Link
{{DEFAULTSORT:Buchwald-Hartwig amination Substitution reactions Name reactions