Benzenamine on:
[Wikipedia]
[Google]
[Amazon]
Aniline is an

 The reduction of nitrobenzene to aniline was first performed by
The reduction of nitrobenzene to aniline was first performed by
 The oxidation of aniline has been heavily investigated, and can result in reactions localized at nitrogen or more commonly results in the formation of new C-N bonds. In alkaline solution,
The oxidation of aniline has been heavily investigated, and can result in reactions localized at nitrogen or more commonly results in the formation of new C-N bonds. In alkaline solution, 
 The reaction to form
The reaction to form 2 C6H5NH2 + CH2O -> CH2(C6H4NH2)2 + H2O
The resulting diamine is the precursor to 4,4'-MDI and related diisocyanates.
 Missing in such an analysis is consideration of solvation. Aniline is, for example, more basic than ammonia in the gas phase, but ten thousand times less so in aqueous solution.
Missing in such an analysis is consideration of solvation. Aniline is, for example, more basic than ammonia in the gas phase, but ten thousand times less so in aqueous solution.
C6H5NH2 + 2 CH3OH -> C6H5N(CH3)2 + 2H2O
''N''-Methylaniline and ''N'',''N''-dimethylaniline are colorless liquids with
 Other uses include
Other uses include 
pp 150–1
In 1932,
International Chemical Safety Card 0011
* {{Authority control Dyes German inventions Hazardous air pollutants IARC Group 3 carcinogens Phenyl compounds
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
with the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwee ...
C6 H5 NH2. Consisting of a phenyl group
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
attached to an amino group
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such a ...
, aniline is the simplest aromatic amine
In organic chemistry, an aromatic amine is an organic compound consisting of an aromatic ring attached to an amine. It is a broad class of compounds that encompasses anilines, but also many more complex aromatic rings and many amine substituents ...
. It is an industrially significant commodity chemical
Commodity chemicals (or bulk commodities or bulk chemicals) are a group of chemicals that are made on a very large scale to satisfy global markets. The average prices of commodity chemicals are regularly published in the chemical trade magazines an ...
, as well as a versatile starting material for fine chemical
In chemistry, fine chemicals are complex, single, pure chemical substances, produced in limited quantities in multipurpose plants by multistep batch chemical or biotechnological processes. They are described by exacting specifications, used f ...
synthesis. Its main use is in the manufacture of precursors to polyurethane
Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethan ...
, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish
Fish are aquatic, craniate, gill-bearing animals that lack limbs with digits. Included in this definition are the living hagfish, lampreys, and cartilaginous and bony fish as well as various extinct related groups. Approximately 95% of li ...
. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.
Relative to benzene, it is electron-rich. It thus participates more rapidly in electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic ni ...
reactions. Likewise, it is also prone to oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Aniline can be diazotized to give a diazonium
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide.
General properti ...
salt, which can then undergo various nucleophilic substitution reactions.
“Aniline” is ultimately from Portuguese ''anil'' which means "the indigo shrub", with suffix ''-ine'' indicating "derived substance".
Like other amines, aniline is both a base (p''K''aH = 4.6) and a nucleophile, although less so than structurally similar aliphatic amines.
Because an early source of the benzene from which they are derived was coal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasi ...
, aniline dyes are also called coal tar dyes.
Structure

Aryl-N distances
In aniline, the C−N bond length is 1.41 Å, compared to 1.47 Å for cyclohexylamine, indicating partial π-bonding between N and C. The C(aryl)-NH2 distance in anilines is highly sensitive tosubstituent effect
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''side ...
s. This distance is 1.34 Å in 2,4,6-trinitroaniline
2,4,6-Trinitroaniline, C6H4N4O6, abbreviated as TNA and also known as picramide, a nitrated amine. Materials in this group range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, the ...
vs 1.44 Å in 3-methylaniline
There are three isomers of toluidine, which are organic compounds. These isomers are ''o''-toluidine, ''m''-toluidine, and ''p''-toluidine, with the prefixed letter abbreviating, respectively, ''ortho''; ''meta''; and ''para''. All three are aryl ...
.
Pyramidalization
The amine in anilines is a slightly pyramidalized molecule, with hybridization of the nitrogen somewhere between sp3 and sp2. The nitrogen is described as having high p character. The amino group in aniline is flatter (i.e., it is a "shallower pyramid") than that in an aliphatic amine, owing to conjugation of thelone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
with the aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
substituent. The observed geometry reflects a compromise between two competing factors: 1) stabilization of the N lone pair in an orbital with significant s character favors pyramidalization (orbitals with s character are lower in energy), while 2) delocalization
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly diff ...
of the N lone pair into the aryl ring favors planarity (a lone pair in a pure p orbital gives the best overlap with the orbitals of the benzene ring π system).
Consistent with these factors, substituted anilines with electron donating groups are more pyramidalized, while those with electron withdrawing groups are more planar. In the parent aniline, the lone pair is approximately 12% s character, corresponding to sp7.3 hybridization. (For comparison, alkylamines generally have lone pairs in orbitals that are close to sp3.)
The pyramidalization angle between the C–N bond and the bisector of the H–N–H angle is 142.5°. For comparison, in more strongly pyramidal methylamine, this value is ~125°, while that of formamide has an angle of 180°.
Production
Industrial aniline production involves two steps. First,benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
is nitrated
In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and ...
with a concentrated mixture of nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
and sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
at 50 to 60 °C to yield nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor t ...
. The nitrobenzene is then hydrogenated
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic co ...
(typically at 200–300 °C) in the presence of metal catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s:
:Nikolay Zinin Nikolay Nikolaevich Zinin (russian: link=no, Никола́й Никола́евич Зи́нин; 25 August 1812, in Shusha – 18 February 1880, in Saint Petersburg) was a Russian organic chemist.
Life
He studied at the University of Kazan where ...
in 1842, using inorganic sulfide as a reductant (Zinin reaction
Zinin reaction or Zinin reduction was discovered by a Russian organic chemist Nikolay Zinin (Russian: Николай Николаевич Зинин) (25 August 1812, Shusha – 18 February 1880, Saint Petersburg). This reaction involves conversion ...
). The reduction of nitrobenzene to aniline was also performed as part of reductions by Antoine Béchamp
Pierre Jacques Antoine Béchamp (16 October 1816 – 15 April 1908) was a French scientist now best known for breakthroughs in applied organic chemistry and for a bitter rivalry with Louis Pasteur.
Béchamp developed the Béchamp reduction ...
in 1854, using iron as the reductant (Bechamp reduction
The Béchamp reduction (or Béchamp process) is a chemical reaction that converts aromatic nitro compounds to their corresponding anilines using iron as the reductant..
:
This reaction was once a major route to aniline, but catalytic hydrogenati ...
).
Aniline can alternatively be prepared from ammonia and phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it req ...
derived from the cumene process
The cumene process (cumene-phenol process, Hock process) is an industrial process for synthesizing phenol and acetone from benzene and propylene. The term stems from cumene (isopropyl benzene), the intermediate material during the process. It was i ...
.
In commerce, three brands of aniline are distinguished: aniline oil for blue, which is pure aniline; aniline oil for red, a mixture of equimolecular quantities of aniline and ortho- and para-toluidine
There are three isomers of toluidine, which are organic compounds. These isomers are ''o''-toluidine, ''m''-toluidine, and ''p''-toluidine, with the prefixed letter abbreviating, respectively, ''ortho''; ''meta''; and ''para''. All three are aryl ...
s; and aniline oil for safranine
Safranin (Safranin O or basic red 2) is a biological stain used in histology and cytology. Safranin is used as a counterstain in some staining protocols, colouring cell nuclei red. This is the classic counterstain in both Gram stains and endospo ...
, which contains aniline and ortho-toluidine
There are three isomers of toluidine, which are organic compounds. These isomers are ''o''-toluidine, ''m''-toluidine, and ''p''-toluidine, with the prefixed letter abbreviating, respectively, ''ortho''; ''meta''; and ''para''. All three are aryl ...
and is obtained from the distillate
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heat ...
(échappés) of the fuchsine
Fuchsine (sometimes spelled fuchsin) or rosaniline hydrochloride is a magenta dye with chemical formula C20H19N3·HCl.
fusion.
Related aniline
derivatives
The derivative of a function is the rate of change of the function's output relative to its input value.
Derivative may also refer to:
In mathematics and economics
* Brzozowski derivative in the theory of formal languages
* Formal derivative, an ...
Many analogues of aniline are known where the phenyl group is further substituted. These include toluidine
There are three isomers of toluidine, which are organic compounds. These isomers are ''o''-toluidine, ''m''-toluidine, and ''p''-toluidine, with the prefixed letter abbreviating, respectively, ''ortho''; ''meta''; and ''para''. All three are aryl ...
s, xylidine
Xylidine can refer to any of the six isomers of xylene amine, or any mixture of them.
The chemical formula of xylidines is C8H11N or, more descriptively, (CH3)2C6H3NH2. The CAS number for the isomer mixture is . They are colorless solids or liqui ...
s, chloroaniline Chloroaniline may refer to any of three isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomeri ...
s, aminobenzoic acid Aminobenzoic acid (a benzoic acid with an amino group) can refer to:
* 4-Aminobenzoic acid (''p''-aminobenzoic acid or ''para''-aminobenzoic acid)
* 3-Aminobenzoic acid (''m''-aminobenzoic acid or ''meta''-aminobenzoic acid)
* 2-aminobenzoic acid ...
s, nitroanilines, and many others. They often are prepared by nitration of the substituted aromatic compounds followed by reduction. For example, this approach is used to convert toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) at ...
into toluidines and chlorobenzene
Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.
Uses
Historical
The major use of chlorob ...
into 4-chloroaniline. Alternatively, using Buchwald-Hartwig coupling or Ullmann reaction approaches, aryl halides can be aminated with aqueous or gaseous ammonia.
Reactions
The chemistry of aniline is rich because the compound has been cheaply available for many years. Below are some classes of its reactions.Oxidation
 The oxidation of aniline has been heavily investigated, and can result in reactions localized at nitrogen or more commonly results in the formation of new C-N bonds. In alkaline solution,
The oxidation of aniline has been heavily investigated, and can result in reactions localized at nitrogen or more commonly results in the formation of new C-N bonds. In alkaline solution, azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of simi ...
results, whereas arsenic acid
Arsenic acid or trihydrogen arsenate is the chemical compound with the formula . More descriptively written as , this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as ...
produces the violet-coloring matter violaniline. Chromic acid
The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixt ...
converts it into quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds
, whereas uch as benzene or naphthalene
Uch ( pa, ;
ur, ), frequently referred to as Uch Sharīf ( pa, ;
ur, ; ''"Noble Uch"''), is a historic city in the southern part of Pakistan's Punjab province. Uch may have been founded as Alexandria on the Indus, a town founded by Alexand ...
by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double ...chlorate
The chlorate anion has the formula ClO3-. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by ...
s, in the presence of certain metallic salts (especially of vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( pas ...
), give aniline black
Polyaniline (PANI) is a conducting polymer and organic semiconductor of the semi-flexible rod polymer family. The compound has been of interest since the 1980s because of its electrical conductivity and mechanical properties. Polyaniline is one o ...
. Hydrochloric acid and potassium chlorate give chloranil
Chloranil is a quinone with the molecular formula C6Cl4O2. Also known as tetrachloro-1,4-benzoquinone, it is a yellow solid. Like the parent benzoquinone, chloranil is a planar molecule J.-M. Lü, S. V. Rosokha, I. S. Neretin and J. K. Kochi, "Qui ...
. Potassium permanganate
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and , an intensely pink to purple solution.
Potassium permanganate is widely used in the c ...
in neutral solution oxidizes it to nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor t ...
; in alkaline solution to azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of simi ...
, ammonia, and oxalic acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early inve ...
; in acid solution to aniline black. Hypochlorous acid
Hypochlorous acid (HClO, HOCl, or ClHO) is a weak acid that forms when chlorine dissolves in water, and itself partially dissociates, forming hypochlorite, ClO−. HClO and ClO− are oxidizers, and the primary disinfection agents of chlorine so ...
gives 4-aminophenol
4-Aminophenol (or ''para''-aminophenol or ''p''-aminophenol) is an organic compound with the formula H2NC6H4OH. Typically available as a white powder, it is commonly used as a developer for black-and-white film, marketed under the name Rodinal.
R ...
and para-amino diphenylamine
Diphenylamine is an organic compound with the formula (C6H5)2NH. The compound is a derivative of aniline, consisting of an amine bound to two phenyl groups. The compound is a colorless solid, but commercial samples are often yellow due to oxidized ...
. Oxidation with persulfate
A persulfate (sometimes known as peroxysulfate or peroxodisulfate) is a compound containing the anions or . The anion contains one peroxide group per sulfur center, whereas in , the peroxide group bridges the sulfur atoms. In both cases, sulfu ...
affords a variety of polyaniline
Polyaniline (PANI) is a conducting polymer and organic semiconductor of the semi-flexible rod polymer family. The compound has been of interest since the 1980s because of its electrical conductivity and mechanical properties. Polyaniline is one of ...
s. These polymers exhibit rich redox and acid-base properties.
Electrophilic reactions at ortho- and para- positions
Likephenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it req ...
s, aniline derivatives are highly susceptible to electrophilic substitution
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a functional group in a compound, which is typically, but not always, aromatic. Aromatic substitution reactions are characteristic of aromatic compounds ...
reactions. Its high reactivity reflects that it is an enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
:
The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and th ...
, which enhances the electron-donating ability of the ring. For example, reaction of aniline with sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
at 180 °C produces sulfanilic acid
Sulfanilic acid (4-aminobenzenesulfonic acid) is an organic compound with the formula H3NC6H4SO3. It is an off-white solid. It is a zwitterion, which explains its high melting point. It is a common building block in organic chemistry."Sulphanili ...
, .
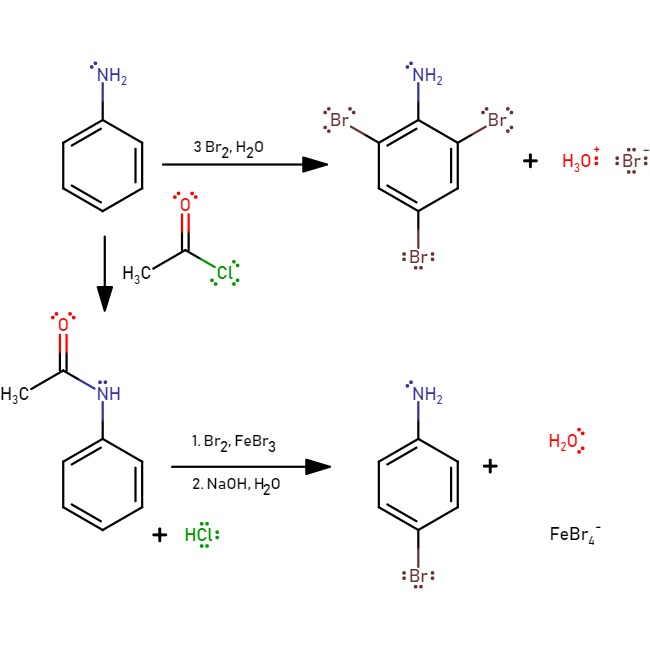
If bromine water is added to aniline, the bromine water
Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (Br2) dissolved in water (H2O). It is often used as a reactive in chemical assays of recognition for substances which react with bromine in an aqueous environment wi ...
is decolourised and a white precipitate
In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a super-saturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading ...
of 2,4,6-tribromoaniline is formed. To generate the mono-substituted product, a protection with acetyl chloride is required:
 The reaction to form
The reaction to form 4-bromoaniline
4-Bromoaniline is a compound where an aniline molecule is substituted with a bromine atom on the ''para'' position. Commercially available, this compound may be used as a building block, e.g. in the preparation of p-bromobiphenyl
Biphenyl (als ...
is to protect the amine with acetyl chloride, then hydrolyse back to reform aniline.
The largest scale industrial reaction of aniline involves its alkylation with formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section F ...
. An idealized equation is shown:
:Reactions at nitrogen
Basicity
Aniline is a weak base.Aromatic amine
In organic chemistry, an aromatic amine is an organic compound consisting of an aromatic ring attached to an amine. It is a broad class of compounds that encompasses anilines, but also many more complex aromatic rings and many amine substituents ...
s such as aniline are, in general, much weaker bases than aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or ...
amines. Aniline reacts with strong acids to form the anilinium
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting ...
(or phenylammonium) ion ().
Traditionally, the weak basicity of aniline is attributed to a combination of inductive effect from the more electronegative sp2 carbon and resonance effects, as the lone pair on the nitrogen is partially delocalized into the pi system of the benzene ring. (see the picture below):
Acylation
Aniline reacts withacyl chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
s such as acetyl chloride
Acetyl chloride (CH3COCl) is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl.
Synthesis
On ...
to give amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
s. The amides formed from aniline are sometimes called anilide
Anilides (or phenylamides) are a class of chemical compounds, which are amide derivatives of aniline.
Preparation
Aniline reacts with acyl chlorides or carboxylic anhydrides to give anilides. For example, reaction of aniline with acetyl chloride ...
s, for example is acetanilide
Acetanilide is an odourless solid chemical of leaf or flake-like appearance. It is also known as ''N''-phenylacetamide, acetanil, or acetanilid, and was formerly known by the trade name Antifebrin.
Preparation and properties
Acetanilide can be ...
. At high temperatures aniline and carboxylic acids react to give the anilides.
''N''-Alkylation
''N''-Methylation of aniline withmethanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
at elevated temperatures over acid catalysts
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
gives ''N''-methylaniline and ''N'',''N''-dimethylaniline:
:boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
s of 193–195 °C and 192 °C, respectively. These derivatives are of importance in the color industry. Aniline combines directly with alkyl iodide
Organoiodine compounds are organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for he ...
s to form secondary and tertiary amines.
Carbon disulfide derivatives
Boiled withcarbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non ...
, it gives sulfocarbanilide (diphenylthiourea
Thiourea () is an organosulfur compound with the formula and the structure . It is structurally similar to urea (), except that the oxygen atom is replaced by a sulfur atom (as implied by the ''thio-'' prefix); however, the properties of urea ...
) (), which may be decomposed into phenyl isothiocyanate
In organic chemistry, isothiocyanate is the functional group , formed by substituting the oxygen in the isocyanate group with a sulfur. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinol ...
(), and triphenyl guanidine
Guanidine is the compound with the formula HNC(NH2)2. It is a colourless solid that dissolves in polar solvents. It is a strong base that is used in the production of plastics and explosives. It is found in urine predominantly in patients experie ...
().
Diazotization
Aniline and its ring-substituted derivatives react withnitrous acid
Nitrous acid (molecular formula ) is a weak and monoprotic acid known only in Solution (chemistry), solution, in the gas phase and in the form of nitrite () salts. Nitrous acid is used to make diazonium salts from amines. The resulting diazoni ...
to form diazonium salt
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide.
General properti ...
s. Through these intermediates, the amine group can be converted to a hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
(), nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix ''cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including met ...
(), or halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
group (, where X is a halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
) via Sandmeyer reaction
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts.
It is an example of a radical-nucleophilic aromatic substitution. The Sandmeyer reaction provides ...
s. This diazonium salt can also be reacted with and phenol to produce a dye known as benzeneazophenol, in a process called ''coupling
A coupling is a device used to connect two shafts together at their ends for the purpose of transmitting power. The primary purpose of couplings is to join two pieces of rotating equipment while permitting some degree of misalignment or end mov ...
''.
The reaction of converting primary aromatic amine into diazonium salt is called diazotisation.
In this reaction primary aromatic amine reacts with sodium nitrile and with 2 moles of HCl which is known as Ice cold mixture because the temperature use to be 0.5 °C and it forms benzene diazonium salt as major product and water and sodium chloride.
Other reactions
It reacts with nitrobenzene to producephenazine
Phenazine is an organic compound with the formula (C6H4)2N2. It is a dibenzo annulation, annulated pyrazine, and the parent substance of many dyestuffs, such as the toluylene red, indulines, and safranines (and the closely related eurhodines). Phe ...
in the Wohl-Aue reaction. Hydrogenation gives cyclohexylamine
Cyclohexylamine is an organic compound, belonging to the aliphatic amine class. It is a colorless liquid, although, like many amines, samples are often colored due to contaminants. It has a fishy odor and is miscible with water. Like other amines, ...
.
Being a standard reagent in laboratories, aniline is used for many niche reactions. Its acetate is used in the aniline acetate test
The aniline acetate test is a chemical test for the presence of certain carbohydrates, in which they are converted to furfural with hydrochloric acid, which reacts with aniline acetate to produce a bright pink color. Pentoses give a strong reactio ...
for carbohydrates, identifying pentoses by conversion to furfural
Furfural is an organic compound with the formula C4H3OCHO. It is a colorless liquid, although commercial samples are often brown. It has an aldehyde group attached to the 2-position of furan. It is a product of the dehydration of sugars, as occurs ...
. It is used to stain neural RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
blue in the Nissl stain
Franz Alexander Nissl (9 September 1860, in Frankenthal – 11 August 1919, in Munich) was a German psychiatrist and medical researcher. He was a noted neuropathologist.
Early life
Nissl was born in Frankenthal to Theodor Nissl and Maria Haas ...
.
Uses
Aniline is predominantly used for the preparation of methylenedianiline and related compounds by condensation with formaldehyde. The diamines are condensed withphosgene
Phosgene is the organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. Phosgene is a valued and important industrial building block, espe ...
to give methylene diphenyl diisocyanate
Methylene diphenyl diisocyanate (MDI) is an aromatic diisocyanate. Three isomers are common, varying by the positions of the isocyanate groups around the rings: 2,2′-MDI, 2,4′-MDI, and 4,4′-MDI. The 4,4′ isomer is most widely used, and i ...
, a precursor to urethane polymers.
:rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, and ...
processing chemicals (9%), herbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page fo ...
s (2%), and dyes and pigments (2%). As additives to rubber, aniline derivatives such as phenylenediamine
Phenylenediamine may refer to:
* ''o''-phenylenediamine or OPD, a chemical compound C6H4(NH2)2
* ''m''-phenylenediamine or MPD, a chemical compound C6H4(NH2)2
* ''p''-phenylenediamine or PPD, a chemical compound C6H4(NH2)2
* ''N,N''-dimethyl-''p'' ...
s and diphenylamine
Diphenylamine is an organic compound with the formula (C6H5)2NH. The compound is a derivative of aniline, consisting of an amine bound to two phenyl groups. The compound is a colorless solid, but commercial samples are often yellow due to oxidized ...
, are antioxidants. Illustrative of the drugs prepared from aniline is paracetamol
Paracetamol, also known as acetaminophen, is a medication used to treat fever and mild to moderate pain. Common brand names include Tylenol and Panadol.
At a standard dose, paracetamol only slightly decreases body temperature; it is inferior ...
(acetaminophen, Tylenol Tylenol may refer to:
* Paracetamol (acetaminophen), a medication used to treat pain and fever
* Tylenol (brand)
Tylenol () is a brand of medication, advertised for reducing pain, reducing fever, and relieving the symptoms of allergies, cold, ...
). The principal use of aniline in the dye industry is as a precursor to indigo
Indigo is a deep color close to the color wheel blue (a primary color in the RGB color space), as well as to some variants of ultramarine, based on the ancient dye of the same name. The word "indigo" comes from the Latin word ''indicum'', m ...
, the blue of blue jeans
Jeans are a type of pants or trousers made from denim or dungaree cloth. Often the term "jeans" refers to a particular style of trousers, called "blue jeans", with copper-riveted pockets which were invented by Jacob W. Davis in 1871 and paten ...
.

History
Aniline was first isolated in 1826 byOtto Unverdorben
Otto Unverdorben (13 October 1806 – 28 November 1873) was a German chemist and merchant who was born in Dahme/Marke. After completing his schooling in Dresden, he studied chemistry at Halle, Leipzig and Berlin.
In 1826 at the age of 20, Unver ...
by destructive distillation
Destruction may refer to:
Concepts
* Destruktion, a term from the philosophy of Martin Heidegger
* Destructive narcissism, a pathological form of narcissism
* Self-destructive behaviour, a widely used phrase that ''conceptualises'' certain kind ...
of indigo
Indigo is a deep color close to the color wheel blue (a primary color in the RGB color space), as well as to some variants of ultramarine, based on the ancient dye of the same name. The word "indigo" comes from the Latin word ''indicum'', m ...
. He called it ''Crystallin''. In 1834, Friedlieb Runge isolated a substance from coal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasi ...
that turned a beautiful blue color when treated with chloride of lime
Calcium hypochlorite is an inorganic compound with formula Ca(OCl)2. It is the main active ingredient of commercial products called bleaching powder, chlorine powder, or chlorinated lime, used for water treatment and as a bleaching agent. This ...
. He named it ''kyanol'' or ''cyanol''. In 1840, Carl Julius Fritzsche
Carl Julius Fritzsche (17 October 1808 in Neustadt in Sachsen, Neustadt – 8 June 1871) was a German pharmacist and chemist. He was a nephew of pharmacist Friedrich Adolph August Struve (1781–1840).
After five years spent working at his unc ...
(1808–1871) treated indigo with caustic potash
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which explo ...
and obtained an oil that he named ''aniline'', after an indigo-yielding plant, anil (''Indigofera suffruticosa
''Indigofera suffruticosa'', commonly known as Guatemalan indigo, small-leaved indigo (Sierra Leone), West Indian indigo, wild indigo, and anil, is a flowering plant in the pea family, Fabaceae.
''Anil'' is native to the subtropical and tropical ...
''). In 1842, Nikolay Nikolaevich Zinin reduced nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor t ...
and obtained a base that he named ''benzidam''. In 1843, August Wilhelm von Hofmann
August Wilhelm von Hofmann (8 April 18185 May 1892) was a German chemist who made considerable contributions to organic chemistry. His research on aniline helped lay the basis of the aniline-dye industry, and his research on coal tar laid the g ...
showed that these were all the same substance, known thereafter as ''phenylamine'' or ''aniline''.
Synthetic dye industry
In 1856, while trying to synthesisequinine
Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to ''Plasmodium falciparum'' that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg cr ...
, von Hofmann's student William Henry Perkin
Sir William Henry Perkin (12 March 1838 – 14 July 1907) was a British chemist and entrepreneur best known for his serendipitous discovery of the first commercial synthetic organic dye, mauveine, made from aniline. Though he failed in trying ...
discovered mauveine
Mauveine, also known as aniline purple and Perkin's mauve, was one of the first synthetic dyes. It was discovered serendipitously by William Henry Perkin in 1856 while he was attempting to synthesise the phytochemical quinine for the treatment of m ...
and went into industry producing the first commercial synthetic Synthetic things are composed of multiple parts, often with the implication that they are artificial. In particular, 'synthetic' may refer to:
Science
* Synthetic chemical or compound, produced by the process of chemical synthesis
* Synthetic o ...
dye. Other aniline dyes followed, such as fuchsin
Fuchsine (sometimes spelled fuchsin) or rosaniline hydrochloride is a magenta dye with chemical formula C20H19N3·HCl.
, safranin
Safranin (Safranin O or basic red 2) is a biological stain used in histology and cytology. Safranin is used as a counterstain in some staining protocols, colouring cell nuclei red. This is the classic counterstain in both Gram stains and endospo ...
, and induline Induline is a dye of blue, bluish-red or black shades. Induline consists of a mixture of several intensely colored species, so the name is often indulines. It was one of the first synthetic dyes, discovered in 1863 by J. Dale and Heinrich Caro. The ...
. At the time of mauveine's discovery, aniline was expensive. Soon thereafter, applying a method reported in 1854 by Antoine Béchamp
Pierre Jacques Antoine Béchamp (16 October 1816 – 15 April 1908) was a French scientist now best known for breakthroughs in applied organic chemistry and for a bitter rivalry with Louis Pasteur.
Béchamp developed the Béchamp reduction ...
, it was prepared "by the ton". The Béchamp reduction enabled the evolution of a massive dye industry in Germany. Today, the name of BASF
BASF Societas Europaea, SE () is a German multinational corporation, multinational chemical company and the List of largest chemical producers, largest chemical producer in the world. Its headquarters is located in Ludwigshafen, Germany.
The ...
, originally ''Badische Anilin- und Soda-Fabrik'' (English: Baden
Baden (; ) is a historical territory in South Germany, in earlier times on both sides of the Upper Rhine but since the Napoleonic Wars only East of the Rhine.
History
The margraves of Baden originated from the House of Zähringen. Baden is ...
Aniline and Soda
Soda or SODA may refer to:
Chemistry
* Some chemical compounds containing sodium
** Sodium carbonate, washing soda or soda ash
** Sodium bicarbonate, baking soda
** Sodium hydroxide, caustic soda
** Sodium oxide, an alkali metal oxide
* Sod ...
Factory), now the largest chemical supplier, echoes the legacy of the synthetic dye industry, built via aniline dyes and extended via the related azo dye
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C-N=N ...
s. The first azo dye was aniline yellow
Aniline Yellow is a yellow azo dye and an aromatic amine. It is a derivative of azobenzene. It has the appearance of an orange powder.
Aniline Yellow was the first azo dye. it was first produced in 1861 by C. Mene. The second azo dye was Bismarc ...
.
Developments in medicine
In the late 19th century, derivatives of aniline such asacetanilide
Acetanilide is an odourless solid chemical of leaf or flake-like appearance. It is also known as ''N''-phenylacetamide, acetanil, or acetanilid, and was formerly known by the trade name Antifebrin.
Preparation and properties
Acetanilide can be ...
and phenacetin
Phenacetin (acetophenetidin, ''N''-(4-ethoxyphenyl)acetamide) is a pain-relieving and fever-reducing drug, which was widely used following its introduction in 1887. It was withdrawn from medicinal use as dangerous from the 1970s (e.g., withdrawn ...
emerged as analgesic
An analgesic drug, also called simply an analgesic (American English), analgaesic (British English), pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain (that is, analgesia or pain management). It ...
drugs, with their cardiac-suppressive side effects
In medicine, a side effect is an effect, whether therapeutic or adverse, that is secondary to the one intended; although the term is predominantly employed to describe adverse effects, it can also apply to beneficial, but unintended, consequence ...
often countered with caffeine
Caffeine is a central nervous system (CNS) stimulant of the methylxanthine class. It is mainly used recreationally as a cognitive enhancer, increasing alertness and attentional performance. Caffeine acts by blocking binding of adenosine t ...
. During the first decade of the 20th century, while trying to modify synthetic dyes to treat African sleeping sickness
African trypanosomiasis, also known as African sleeping sickness or simply sleeping sickness, is an insect-borne parasitic infection of humans and other animals. It is caused by the species '' Trypanosoma brucei''. Humans are infected by two ty ...
, Paul Ehrlich
Paul Ehrlich (; 14 March 1854 – 20 August 1915) was a Nobel Prize-winning German physician and scientist who worked in the fields of hematology, immunology, and antimicrobial chemotherapy. Among his foremost achievements were finding a cure ...
– who had coined the term ''chemotherapy
Chemotherapy (often abbreviated to chemo and sometimes CTX or CTx) is a type of cancer treatment that uses one or more anti-cancer drugs (chemotherapeutic agents or alkylating agents) as part of a standardized chemotherapy regimen. Chemotherap ...
'' for his '' magic bullet'' approach to medicine – failed and switched to modifying Béchamp's atoxyl
Arsanilic acid, also known as aminophenyl arsenic acid or aminophenyl arsonic acid, is an organoarsenic compound, an amino derivative of phenylarsonic acid whose amine group is in the 4-position. A crystalline powder introduced medically in the l ...
, the first organic arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but ...
al drug, and serendipitously obtained a treatment for syphilis
Syphilis () is a sexually transmitted infection caused by the bacterium ''Treponema pallidum'' subspecies ''pallidum''. The signs and symptoms of syphilis vary depending in which of the four stages it presents (primary, secondary, latent, an ...
– salvarsan
Arsphenamine, also known as Salvarsan or compound 606, is a drug that was introduced at the beginning of the 1910s as the first effective treatment for syphilis, relapsing fever, and African trypanosomiasis.
This organoarsenic compound was the fi ...
– the first successful chemotherapy agent. Salvarsan's targeted microorganism, not yet recognized as a bacterium, was still thought to be a parasite, and medical bacteriologists, believing that bacteria were not susceptible to the chemotherapeutic approach, overlooked Alexander Fleming
Sir Alexander Fleming (6 August 1881 – 11 March 1955) was a Scottish physician and microbiologist, best known for discovering the world's first broadly effective antibiotic substance, which he named penicillin. His discovery in 1928 of w ...
's report in 1928 on the effects of penicillin
Penicillins (P, PCN or PEN) are a group of β-lactam antibiotics originally obtained from ''Penicillium'' moulds, principally '' P. chrysogenum'' and '' P. rubens''. Most penicillins in clinical use are synthesised by P. chrysogenum using ...
.D J Th Wagener, ''The History of Oncology'' (Houten: Springer, 2009)pp 150–1
In 1932,
Bayer
Bayer AG (, commonly pronounced ; ) is a German multinational corporation, multinational pharmaceutical and biotechnology company and one of the largest pharmaceutical companies in the world. Headquartered in Leverkusen, Bayer's areas of busi ...
sought medical applications of its dyes. Gerhard Domagk
Gerhard Johannes Paul Domagk (; 30 October 1895 – 24 April 1964) was a German pathologist and bacteriologist. He is credited with the discovery of sulfonamidochrysoidine (KL730) as an antibiotic for which he received the 1939 Nobel Prize in Phy ...
identified as an antibacterial
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
a red azo dye, introduced in 1935 as the first antibacterial drug, prontosil
Prontosil is an antibacterial drug of the sulfonamide group. It has a relatively broad effect against gram-positive cocci but not against enterobacteria. One of the earliest antimicrobial drugs, it was widely used in the mid-20th century but is ...
, soon found at Pasteur Institute
The Pasteur Institute (french: Institut Pasteur) is a French non-profit private foundation dedicated to the study of biology, micro-organisms, diseases, and vaccines. It is named after Louis Pasteur, who invented pasteurization and vaccines f ...
to be a prodrug
A prodrug is a medication or compound that, after intake, is metabolized (i.e., converted within the body) into a pharmacologically active drug. Instead of administering a drug directly, a corresponding prodrug can be used to improve how the drug ...
degraded ''in vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, and ...
'' into sulfanilamide
Sulfanilamide (also spelled sulphanilamide) is a sulfonamide antibacterial drug. Chemically, it is an organic compound consisting of an aniline derivatized with a sulfonamide group. Powdered sulfanilamide was used by the Allies in World War II ...
– a colorless intermediate for many, highly colorfast
Colour fastness is a term—used in the dyeing of textile materials—that characterizes a material's colour's resistance to fading or running. Colour fastness is the property of dyes and it is directly proportional to the binding force between pho ...
azo dyes – already with an expired patent, synthesized in 1908 in Vienna by the researcher Paul Gelmo Paul Josef Jakob Gelmo (17 December 1879 – 22 October 1961) was an Austrian chemist who worked on synthetic dyes and discovered sulfanilamide in 1908, although their antibiotic properties were discovered only in 1932.
Gelmo was born in Vienna an ...
for his doctoral research. By the 1940s, over 500 related sulfa drug
Sulfonamide is a functional group (a part of a molecule) that is the basis of several groups of drugs, which are called sulphonamides, sulfa drugs or sulpha drugs. The original antibacterial sulfonamides are synthetic (nonantibiotic) antimi ...
s were produced. Medications in high demand during World War II
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the vast majority of the world's countries—including all of the great powers—forming two opposin ...
(1939–45), these first ''miracle drugs'', chemotherapy of wide effectiveness, propelled the American pharmaceutics industry. In 1939, at Oxford University
Oxford () is a city in England. It is the county town and only city of Oxfordshire. In 2020, its population was estimated at 151,584. It is north-west of London, south-east of Birmingham and north-east of Bristol. The city is home to the ...
, seeking an alternative to sulfa drugs, Howard Florey
Howard Walter Florey, Baron Florey (24 September 189821 February 1968) was an Australian pharmacologist and pathologist who shared the Nobel Prize in Physiology or Medicine in 1945 with Sir Ernst Chain and Sir Alexander Fleming for his role in ...
developed Fleming's penicillin into the first systemic antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
drug, penicillin G
Benzylpenicillin, also known as penicillin G (PenG) or BENPEN, and in military slang "Peanut Butter Shot" is an antibiotic used to treat a number of bacterial infections. This includes pneumonia, strep throat, syphilis, necrotizing enterocolitis ...
. (Gramicidin
Gramicidin, also called gramicidin D, is a mix of ionophoric antibiotics, gramicidin A, B and C, which make up about 80%, 5%, and 15% of the mix, respectively. Each has 2 isoforms, so the mix has 6 different types of gramicidin molecules. They c ...
, developed by René Dubos
René Jules Dubos (February 20, 1901 – February 20, 1982) was a French-American microbiologist, experimental pathologist, environmentalist, humanist, and winner of the Pulitzer Prize for General Non-Fiction for his book ''So Human An Animal ...
at Rockefeller Institute in 1939, was the first antibiotic, yet its toxicity restricted it to topical
A topical medication is a medication that is applied to a particular place on or in the body. Most often topical medication means application to body surfaces such as the skin or mucous membranes to treat ailments via a large range of classes ...
use.) After World War II, Cornelius P. Rhoads
Cornelius Packard "Dusty" Rhoads (June 9, 1898 – August 13, 1959) was an American pathologist, oncologist, and hospital administrator who was involved in a racist scandal and subsequent whitewashing in the 1930s. Beginning in 1940, he served a ...
introduced the chemotherapeutic approach to cancer treatment.
Rocket fuel
Some early American rockets, such as theAerobee
The Aerobee rocket was one of the United States' most produced and productive sounding rockets. Developed by the Aerojet Corporation, the Aerobee was designed to combine the altitude and launching capability of the V-2 with the cost effectiven ...
and WAC Corporal
The WAC Corporal was the first sounding rocket developed in the United States and the first vehicle to achieve hypersonic speeds. It was an offshoot of the Corporal program, that was started by a partnership between the United States Army Ordn ...
, used a mixture of aniline and furfuryl alcohol
Furfuryl alcohol is an organic compound containing a furan substituted with a hydroxymethyl group. It is a colorless liquid, but aged samples appear amber. It possesses a faint odor of burning and a bitter taste. It is miscible with but unstabl ...
as a fuel, with nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
as an oxidizer. The combination is hypergolic
A hypergolic propellant is a rocket propellant combination used in a rocket engine, whose components spontaneously ignite when they come into contact with each other.
The two propellant components usually consist of a fuel and an oxidizer. The ...
, igniting on contact between fuel and oxidizer. It is also dense, and can be stored for extended periods. Aniline was later replaced by hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
.Brian Burnell. 2016. http://www.nuclear-weapons.info/cde.htm#Corporal SSM
Toxicology and testing
Aniline is toxic by inhalation of the vapour, ingestion, or percutaneous absorption.Muir, GD (ed.) 1971, ''Hazards in the Chemical Laboratory'', The Royal Institute of Chemistry, London. TheIARC IARC may refer to:
* International Aerial Robotics Competition
* International Age Rating Coalition
* International Agency for Research on Cancer
* International Arctic Research Center
* Israel Amateur Radio Club
* iArc IARC may refer to:
* Internat ...
lists it in Group 3 Group 3 may refer to:
*Group 3 element, chemical element classification
*Group 3 (racing), FIA classification for auto racing
*Group 3, the third tier of races in worldwide Thoroughbred horse racing
* Group 3 image format, Group 3 & Group 4 are d ...
(''not classifiable as to its carcinogenicity to humans'') due to the limited and contradictory data available. The early manufacture of aniline resulted in increased incidents of bladder cancer, but these effects are now attributed to naphthylamine Naphthylamine can refer to either of two isomeric chemical compounds:
*1-Naphthylamine
1-Naphthylamine is an aromatic amine derived from naphthalene. It can cause bladder cancer (transitional cell carcinoma). It crystallizes in colorless needles w ...
s, not anilines.
Aniline has been implicated as one possible cause of forest dieback
Forest dieback (also "", a German loan word) is a condition in trees or woody plants in which peripheral parts are killed, either by pathogens, parasites or conditions like acid rain, drought, and more. These episodes can have disastrous conseq ...
.Krahl-Urban, B., Papke, H.E., Peters, K. (1988) ''Forest Decline: Cause-Effect Research in the United States of North America and Federal Republic of Germany''. Germany: Assessment Group for Biology, Ecology and Energy of the Julich Nuclear Research Center.
Many methods exist for the detection of aniline.''Basic Analytical Toxicology'' (1995), R. J. Flanagan, S. S. Brown, F. A. de Wolff, R. A. Braithwaite, B. Widdop: World Health Organization
Oxidative DNA damage
Exposure of rats to aniline can elicit a response that is toxic to thespleen
The spleen is an organ found in almost all vertebrates. Similar in structure to a large lymph node, it acts primarily as a blood filter. The word spleen comes .
, including a tumorigenic
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnorm ...
response. Rats exposed to aniline in drinking water, showed a significant increase in oxidative DNA damage
DNA repair is a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as radiation can cause DNA da ...
to the spleen, detected as a 2.8-fold increase in 8-hydroxy-2’-deoxyguanosine (8-OHdG) in their DNA. Although the base excision repair
Base excision repair (BER) is a cellular mechanism, studied in the fields of biochemistry and genetics, that repairs damaged DNA throughout the cell cycle. It is responsible primarily for removing small, non-helix-distorting base lesions from t ...
pathway was also activated, its activity was not sufficient to prevent the accumulation of 8-OHdG. The accumulation of oxidative DNA damages in the spleen following exposure to aniline may increase mutagenic events that underlie tumorigenesis.
Notes
References
*External links
*International Chemical Safety Card 0011
* {{Authority control Dyes German inventions Hazardous air pollutants IARC Group 3 carcinogens Phenyl compounds