Bargellini reaction on:
[Wikipedia]
[Google]
[Amazon]
The Bargellini reaction is a 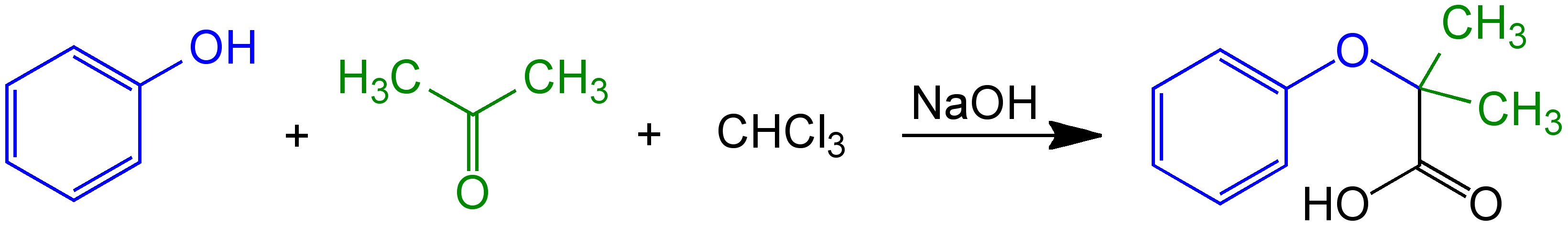 Later, organic chemists have used the reaction as a general method of organic synthesis for highly hindered or bulky morpholinones or piperazinones from ketones (particularly
Later, organic chemists have used the reaction as a general method of organic synthesis for highly hindered or bulky morpholinones or piperazinones from ketones (particularly 
 Reaction mechanism for original Bargellini reaction (1906):
:
Reaction mechanism for original Bargellini reaction (1906):
: Present-day Bargellini reaction used for synthesis of hindered morpholinones or piperazinones from ketones (primarily acetone) and 2-amino-2-methylpropan-1-ol (β-amino alcohols) OR 1,2-diaminopropanes (diamines). The solvent used is dichloromethane (DCM), also known as
Present-day Bargellini reaction used for synthesis of hindered morpholinones or piperazinones from ketones (primarily acetone) and 2-amino-2-methylpropan-1-ol (β-amino alcohols) OR 1,2-diaminopropanes (diamines). The solvent used is dichloromethane (DCM), also known as  Reaction mechanism for Bargellini reaction:
:
Reaction mechanism for Bargellini reaction:
: The reaction mechanism proceeds when a sterically accessible ketone, usually acetone, is added to a solution of chloroform (trichloromethane) under strong basic conditions, creating a trichloromethide anion by
The reaction mechanism proceeds when a sterically accessible ketone, usually acetone, is added to a solution of chloroform (trichloromethane) under strong basic conditions, creating a trichloromethide anion by
chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
discovered in 1906 by Italian chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties. Chemists carefully describe t ...
Guido Bargellini. The original reaction was a mixture of the reagents phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it ...
, chloroform, and acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscib ...
in the presence of a sodium hydroxide solution. Prior to Bargellini's research, the product attributed to this multi-component reaction In chemistry, a multi-component reaction (or MCR), sometimes referred to as a "Multi-component Assembly Process" (or MCAP), is a chemical reaction where three or more compounds
react to form a single product. By definition, multicomponent reaction ...
(MCR) had been described as a phenol derivative in chemistry texts at the time. However, Bargellini demonstrated that a carboxylic acid derivative was actually the correct structure.
 Later, organic chemists have used the reaction as a general method of organic synthesis for highly hindered or bulky morpholinones or piperazinones from ketones (particularly
Later, organic chemists have used the reaction as a general method of organic synthesis for highly hindered or bulky morpholinones or piperazinones from ketones (particularly acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscib ...
) and either β-amino alcohols or diamines.
History
Guido Bargellini was a disciple of Hermann Emil Louis Fischer, the German chemist and Nobel laureate famous for the eponymous Fischer esterification reaction. Bargellini did his post-doctoral lab research in Fischer's laboratory. He spent most of his career as a chemist at the University of Rome. His interest incoumarins
Coumarin () or 2''H''-chromen-2-one is an aromatic organic chemical compound with formula . Its molecule can be described as a benzene molecule with two adjacent hydrogen atoms replaced by a lactone-like chain , forming a second six-membered h ...
, a recently isolated compound at the time, led Bargellini to experiment with multi-component reactions (MCRs) between phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are ...
, chloroform, and acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscib ...
in a solution of a sodium hydroxide. He discovered the structure given to the compound produced a carboxylic acid instead of a phenol as previously thought. In 1894, Link, a German chemist, had published the reaction in ''Chemisches Zentralblatt
''Chemisches Zentralblatt'' is the first and oldest abstracts journal published in the field of chemistry. It covers the chemical literature from 1830 to 1969 and describes therefore the "birth" of chemistry as science, in contrast to alchemy. The ...
'' and patented it. However, he wrote the product was either a ketone or a phenol, specifically he claimed it was a "hydrpxyphenyl hydroxyisopropyl keton" or "hydroxyisobutyrylphenol." When Bargellini conducted the same experiment and began testing the product, the chemical properties could not be from a ketone or a phenol. Instead, he was certain it was a carboxylic acid, specifically an "α-phenoxyisobutyric acid." Link himself experimented with reactions in 1900 that proved his original claim was erroneous, yet it was never changed. Since Bargellini correctly identified the product, its structure and properties, then published his results in the ''Gazzetta Chimica Italiana
''Gazzetta Chimica Italiana'' was an Italian peer-reviewed scientific journal in chemistry. It was established in 1871 by the Italian Chemical Society (''Società Chimica Italiana''), but in 1998 publication ceased and it was merged with some o ...
'', the reaction was named after him.
However, the importance of the reaction in organic synthesis and later the pharmaceutical industry has made it important historically. Since the reaction is relatively easy to perform—the reagents being readily available—many other almost identical reactions were named in the decades after. This discovery led the way for new transformation reaction, the presently-established Bargellini-type reactions, that has been of great importance, specifically in the pharmaceutical industry
The pharmaceutical industry discovers, develops, produces, and markets drugs or pharmaceutical drugs for use as medications to be administered to patients (or self-administered), with the aim to cure them, vaccinate them, or alleviate symptoms. ...
. It also paved the way for later name reactions, like the Jocic–Reeve reaction
In organic chemistry, the Jocic reaction, also called the Jocic–Reeve reaction (named after Zivojin Jocic and Wilkins Reeve) is a name reaction that generates α-substituted carboxylic acids from trichloromethylcarbinols and corresponding nucle ...
and the Corey–Link reaction. The Jocic–Reeve and Corey–Link reactions are almost always featured together with the Bargellini reaction in a MCR. The reaction itself has been modified several times to increase efficiency or produce a modified product.
The adaptability of the reaction is one of its greatest aspects. No decade has gone by without an important addition or twist of the reaction taking place. In the author's own words, "The first phase in the reaction is probably the formation of acetonechloroform--(which may, indeed, be used in place of the chloroform), this being then acted on by sodium hydroxide in presence of acetone, yielding α-hydroxyisobutyric acid, which, with the phenol, gives α-phenoxyisobutyric acid. The chloroform may also be replaced by bromoform
Bromoform (CHBr3) is a brominated organic solvent, colorless liquid at room temperature, with a high refractive index, very high density, and sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluorofor ...
, bromal, chloral
Chloral, also known as trichloroacetaldehyde or trichloroethanal, is the organic compound with the formula Cl3CCHO. This aldehyde is a colourless oily liquid that is soluble in a wide range of solvents. It reacts with water to form chloral hydrate ...
, or carbon tetrachloride or tetrabromide." Most textbooks describe the reaction as a way to make morpholinones or piperazinones, but it use extend much farther than that.
One hundred years later, the Bargellini reaction itself was used for the condensation of coumarin
Coumarin () or 2''H''-chromen-2-one is an aromatic organic chemical compound with formula . Its molecule can be described as a benzene molecule with two adjacent hydrogen atoms replaced by a lactone-like chain , forming a second six-membered h ...
s, an ironic twist to the history of the reaction since this was Bargellini's primary compound of interest and his own named reaction produced it.
Reactions and reaction mechanisms
The original Bargellini reaction (1906): : Reaction mechanism for original Bargellini reaction (1906):
:
Reaction mechanism for original Bargellini reaction (1906):
: Present-day Bargellini reaction used for synthesis of hindered morpholinones or piperazinones from ketones (primarily acetone) and 2-amino-2-methylpropan-1-ol (β-amino alcohols) OR 1,2-diaminopropanes (diamines). The solvent used is dichloromethane (DCM), also known as
Present-day Bargellini reaction used for synthesis of hindered morpholinones or piperazinones from ketones (primarily acetone) and 2-amino-2-methylpropan-1-ol (β-amino alcohols) OR 1,2-diaminopropanes (diamines). The solvent used is dichloromethane (DCM), also known as methylene chloride
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with ...
with a benzyltriethylammonium chloride catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. The solvent and catalyst are frequently changed when using different reagents. Diamine
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term ''diamine'' refers mostly to primary diamines, as those are the most reactive.
In terms of quantities p ...
s tend to give higher product yields than β- amino alcohols, as shown in the two possible scenarios below:
: Reaction mechanism for Bargellini reaction:
:
Reaction mechanism for Bargellini reaction:
: The reaction mechanism proceeds when a sterically accessible ketone, usually acetone, is added to a solution of chloroform (trichloromethane) under strong basic conditions, creating a trichloromethide anion by
The reaction mechanism proceeds when a sterically accessible ketone, usually acetone, is added to a solution of chloroform (trichloromethane) under strong basic conditions, creating a trichloromethide anion by deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
. This forms the corresponding trichloromethyl carbinol or - alkoxide, in a similar way to the Grignard reaction
The Grignard reaction () is an organometallic chemical reaction in which alkyl, allyl, vinyl, or aryl-magnesium halides (Grignard reagent) is added to a carbonyl group in an aldehyde or ketone. This reaction is important for the formation of ...
.
This trihalogenated product is subject to addition via a base-induced intramolecular etherification ''gem''-dichloro epoxy. The amine can attack the oxirane
Ethylene oxide is an organic compound with the formula . It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly swe ...
due to formation of tertiary carbocation in a nucleophilic substitution SN1 concerted elimination of one atom of chlorine. The nucleophilic intermediate is highly reactive and regioselective at the α-carbon, resulting in the formation of a α-substituted carboxylic acid chloride.
The final step occurs by nucleophilic acyl substitution and solvolysis
In chemistry, solvolysis is a type of nucleophilic substitution (S1/S2) or elimination reaction, elimination where the nucleophile is a solvent molecule. Characteristic of S1 reactions, solvolysis of a chirality (chemistry), chiral reactant affor ...
, where the amino or hydroxyl group attacks the acid chloride forming the corresponding heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
.Timothy S. Snowden: ''Recent applications of gem-dichloroepoxide intermediates in synthesis.'' In: ''ARKIVOC.'' 2, 2012, S. 24–40 The end product is a carboxylic acid derivative (primarily lactones and amides).
References
External links
* https://pubchem.ncbi.nlm.nih.gov/ * http://www.synarchive.com/named-reactions/Bargellini_Reaction {{Organic reactions Name reactions