|
Bromoform
Bromoform (CHBr3) is a brominated organic solvent, colorless liquid at room temperature, with a high refractive index, very high density, and sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. Bromoform can be prepared by the haloform reaction using acetone and sodium hypobromite, by the electrolysis of potassium bromide in ethanol, or by treating chloroform with aluminium bromide. Currently its main use is as a laboratory reagent. Structure The molecule adopts tetrahedral molecular geometry with C3v symmetry. Uses Only small quantities of bromoform are currently produced industrially in the United States. In the past, it was used as a solvent, sedative and flame retardant, but now it is mainly used as a laboratory reagent, for example as an extraction solvent. Bromoform also has medical uses; injections of bromoform are sometimes used instead of epinephrine to treat severe asthma cases. Bromo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haloform
In chemistry, trihalomethanes (THMs) are chemical compounds in which three of the four hydrogen atoms of methane () are replaced by halogen atoms. Many trihalomethanes find uses in industry as solvents or refrigerants. THMs are also environmental pollutants, and many are considered carcinogenic. Trihalomethanes with all the same halogen atoms are called haloforms. Table of common trihalomethanes Industrial uses Only chloroform has significant applications of the haloforms. In the predominant application, chloroform is required for the production of tetrafluoroethylene, precursor to teflon. Chloroform is fluorinated by reaction with hydrogen fluoride to produce chlorodifluoromethane (R-22). Pyrolysis of chlorodifluoromethane (at 550-750 °C) yields TFE, with difluorocarbene as an intermediate. :CHCl3 + 2 HF -> CHClF2 + 2 HCl :2 CHClF2 -> C2F4 + 2 HCl Refrigerants and solvents Trifluoromethane and chlorodifluoromethane are both used as refrigerants. Trihalomethanes relea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodoform
Iodoform (also known as triiodomethane and, inaccurately, as carbon triiodide) is the organoiodine compound with the chemical formula C H I3. A pale yellow, crystalline, volatile substance, it has a penetrating and distinctive odor (in older chemistry texts, the smell is sometimes referred to as that of hospitals, where the compound is still commonly used) and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant. Structure The molecule adopts tetrahedral molecular geometry with C3v symmetry. Synthesis and reactions The synthesis of iodoform was first described by Georges-Simon Serullas in 1822, by reactions of iodine vapour with steam over red-hot coals, and also by reaction of potassium with ethanolic iodine in the presence of water; and at much the same time independently by John Thomas Cooper. It is synthesized in the haloform reaction by the reaction of iodine and sodium hydroxide with any one of these four kinds of organic compounds: a methyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrabromomethane
Tetrabromomethane, CBr4, also known as carbon tetrabromide, is a carbon bromide. Both names are acceptable under IUPAC nomenclature. Physical properties Tetrabromomethane has two polymorphs: crystalline II or β below 46.9 °C (320.0 K) and crystalline I or α above 46.9 °C. Monoclinic polymorph has space group ''C2/c'' with lattice constants: ''a'' = 20.9, ''b'' = 12.1, ''c'' = 21.2 (.10−1 nm), β = 110.5°.F. Brezina, J. Mollin, R. Pastorek, Z. Sindelar. ''Chemicke tabulky anorganickych sloucenin'' (''Chemical tables of inorganic compounds''). SNTL, 1986. Bond energy of C-Br is 235 kJ.mol−1.N. N. Greenwood, A. Earnshaw. ''Chemie prvku'' (''Chemistry of the Elements''). Informatorium, Prague, 1993. Due to its symmetrically substituted tetrahedral structure, its dipole moment is 0 Debye. Critical temperature is 439 °C (712 K) and critical pressure is 4.26 MPa. Plastic crystallinity The high temperature α phase is known as a plastic crystal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1-Dibromoethane
1,1-Dibromoethane is a clear, slightly brown, flammable chemical compound. It is classified as the organobromine compound, and has the chemical formula CHBr and it is a position isomer of 1,2-Dibromoethane, 1,2-dibromoethane. It is commonly seen in industrial chemistry, where it is used as a fuel additive. It is also used as a grain and soil fumigant for insect control. Synthesis 1,1-Dibromoethane is synthesized through addition of hydrogen bromide onto vinyl bromide with absence of peroxide radical. : Safety 1,1-Dibromoethane is considered as a mild toxic compound, especially with bromines attached as substituents. Bromines on the ethane are strong oxidizing agents. If absorbed through inhalation, 1,1-dibromoethane could potentially cause neuronal effects, tissue damage, and bromism. References {{DEFAULTSORT:Dibromoethane, 1, 1- Bromoalkanes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Dibromoethane
1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine compound with the chemical formula . Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly synthetic. It is a dense colorless liquid with a faint sweet odor, detectable at 10 ppm, is a widely used and sometimes-controversial fumigant. The combustion of 1,2-dibromoethane produces hydrogen bromide gas that is significantly corrosive. Preparation and use It is produced by the reaction of ethylene gas with bromine, in a classic halogen addition reaction: :CH=CH + Br → BrCH–CHBr Historically, 1,2-dibromoethane was used as a component in anti-knock additives in leaded fuels. It reacts with lead residues to generate volatile lead bromides, thereby preventing fouling of the engine with lead deposits. Pesticide It has been used as a pesticide in soil and on various crops. The applications were initiated after the forced retirement of 1,2-dibrom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrabromoethane
Tetrabromoethane (TBE) is a halogenated hydrocarbon, chemical formula C2H2Br4. Although three bromine atoms may bind to one of the carbon atoms creating 1,1,1,2-tetrabromoethane this is not thermodynamically favorable, so in practice tetrabromoethane is equal to 1,1,2,2-tetrabromoethane, where each carbon atom binds two bromine atoms. Uses It has an unusually high density for an organic compound, near 3 g/mL, due largely to the four bromine atoms.Organic based heavy liquids heavyliquids.com TBE is a at , and is used to separate |
FutureFeed
FutureFeed is a seaweed-based feed ingredient for livestock that is currently being developed by a team from Australia's Commonwealth Scientific and Industrial Research Organisation (CSIRO). The primary component of FutureFeed is dried ''Asparagopsis'', a genus of red algae, which has been shown to reduce the methane (CH4) emissions of ruminant livestock by up to 99%. It is added to fodder at feedlots in dosages of 1-2% dietary intake to achieve this result. FutureFeed is currently being developed in collaboration with James Cook University (JCU) and Meat and Livestock Australia (MLA), with the primary goal of scaling for mainstream commercial use. History Historical evidence suggests that farmers in Ancient Greece deliberately grazed cattle near beaches as a result of the productivity benefits it provided. This was also the case for Icelandic farmers in the 18th century. In the early 2010s, Canadian dairy farmer, Joe Dorgan, noticed that cattle in paddocks adjacent to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dibromomethane
Preparation Dibromomethane is prepared commercially from dichloromethane via bromochloromethane: :6 CH2Cl2 + 3 Br2 + 2 Al → 6 CH2BrCl + 2 AlCl3 :CH2Cl2 + HBr → CH2BrCl + HCl The latter route requires aluminium trichloride as a catalyst. The bromochloromethane product from either reaction can further react in a similar manner: :6 CH2BrCl + 3 Br2 + 2 Al → 6 CH2Br2 + 2 AlCl3 :CH2BrCl + HBr → CH2Br2 + HCl In the laboratory, it is prepared from bromoform: :CHBr3 + Na3AsO3 + NaOH → CH2Br2 + Na3AsO4 + NaBr using sodium arsenite and sodium hydroxide. Another way is to prepare it from diiodomethane and bromine. Uses Dibromomethane is used as a solvent, gauge fluid, and in organic synthesis (often as 1H-NMR internal standard). It is a convenient agent for converting catechols to their methylenedioxy derivatives. Natural occurrence It is naturally produced by marine algae and liberated to the oceans. Releasing on soil causes it to evaporate and leach into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
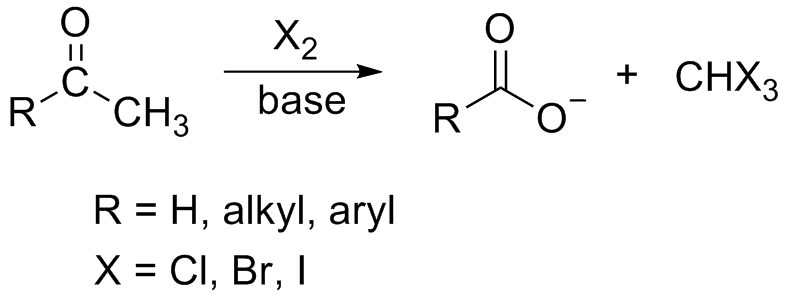
Haloform Reaction
In chemistry, the haloform reaction is a chemical reaction in which a haloform (, where X is a halogen) is produced by the exhaustive halogenation of an acetyl group (, where R can be either a hydrogen atom, an alkyl or an aryl group), in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups () or to produce chloroform (), bromoform (), or iodoform (). Note that fluoroform () can't be prepared in this way. Mechanism In the first step, the halogen dis-proportionates in the presence of hydroxide to give the halide and hypohalite. :Br2 + 2 OH- -> Br- + BrO- + H2O If a secondary alcohol is present, it is oxidized to a ketone by the hypohalite: If a methyl ketone is present, it reacts with the hypohalite in a three-step process: 1. Under basic conditions, the ketone undergoes keto-enol tautomerisation. The enolate undergoes electrophilic attack by the hypohalite (containing a halogen with a formal +1 charge). : 2. When the α(al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society (professional association) in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 34,000 in the UK and a further 8,000 abroad. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symmetry Group
In group theory, the symmetry group of a geometric object is the group of all transformations under which the object is invariant, endowed with the group operation of composition. Such a transformation is an invertible mapping of the ambient space which takes the object to itself, and which preserves all the relevant structure of the object. A frequent notation for the symmetry group of an object ''X'' is ''G'' = Sym(''X''). For an object in a metric space, its symmetries form a subgroup of the isometry group of the ambient space. This article mainly considers symmetry groups in Euclidean geometry, but the concept may also be studied for more general types of geometric structure. Introduction We consider the "objects" possessing symmetry to be geometric figures, images, and patterns, such as a wallpaper pattern. For symmetry of physical objects, one may also take their physical composition as part of the pattern. (A pattern may be specified formally as a scalar field, a funct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sedative
A sedative or tranquilliser is a substance that induces sedation by reducing irritability or excitement. They are CNS depressants and interact with brain activity causing its deceleration. Various kinds of sedatives can be distinguished, but the majority of them affect the neurotransmitter gamma-aminobutyric acid (GABA). In spite of the fact that each sedative acts in its own way, most produce relaxing effects by increasing GABA activity. This group is related to hypnotics. The term ''sedative'' describes drugs that serve to calm or relieve anxiety, whereas the term ''hypnotic'' describes drugs whose main purpose is to initiate, sustain, or lengthen sleep. Because these two functions frequently overlap, and because drugs in this class generally produce dose-dependent effects (ranging from anxiolysis to loss of consciousness) they are often referred to collectively as ''sedative-hypnotic'' drugs. Sedatives can be used to produce an overly-calming effect ( alcohol being the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |