|
Iodoform
Iodoform (also known as triiodomethane and, inaccurately, as carbon triiodide) is the organoiodine compound with the chemical formula C H I3. A pale yellow, crystalline, volatile substance, it has a penetrating and distinctive odor (in older chemistry texts, the smell is sometimes referred to as that of hospitals, where the compound is still commonly used) and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant. Structure The molecule adopts tetrahedral molecular geometry with C3v symmetry. Synthesis and reactions The synthesis of iodoform was first described by Georges-Simon Serullas in 1822, by reactions of iodine vapour with steam over red-hot coals, and also by reaction of potassium with ethanolic iodine in the presence of water; and at much the same time independently by John Thomas Cooper. It is synthesized in the haloform reaction by the reaction of iodine and sodium hydroxide with any one of these four kinds of organic compounds: a methyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodoform
Iodoform (also known as triiodomethane and, inaccurately, as carbon triiodide) is the organoiodine compound with the chemical formula C H I3. A pale yellow, crystalline, volatile substance, it has a penetrating and distinctive odor (in older chemistry texts, the smell is sometimes referred to as that of hospitals, where the compound is still commonly used) and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant. Structure The molecule adopts tetrahedral molecular geometry with C3v symmetry. Synthesis and reactions The synthesis of iodoform was first described by Georges-Simon Serullas in 1822, by reactions of iodine vapour with steam over red-hot coals, and also by reaction of potassium with ethanolic iodine in the presence of water; and at much the same time independently by John Thomas Cooper. It is synthesized in the haloform reaction by the reaction of iodine and sodium hydroxide with any one of these four kinds of organic compounds: a methyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodoform Synthesis
Iodoform (also known as triiodomethane and, inaccurately, as carbon triiodide) is the organoiodine compound with the chemical formula C H I3. A pale yellow, crystalline, volatile substance, it has a penetrating and distinctive odor (in older chemistry texts, the smell is sometimes referred to as that of hospitals, where the compound is still commonly used) and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant. Structure The molecule adopts tetrahedral molecular geometry with C3v symmetry. Synthesis and reactions The synthesis of iodoform was first described by Georges-Simon Serullas in 1822, by reactions of iodine vapour with steam over red-hot coals, and also by reaction of potassium with ethanolic iodine in the presence of water; and at much the same time independently by John Thomas Cooper. It is synthesized in the haloform reaction by the reaction of iodine and sodium hydroxide with any one of these four kinds of organic compounds: a methyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Georges-Simon Serullas
Georges-Simon Serullas (2 November 1774 in Poncin – 25 May 1832 in Paris) was a professor of pharmacy notable for being the first to publish a work on Iodoform, an early antiseptic and disinfectant. Biography He was a professor and head pharmacist at the hospital of Val-de-Grâce; professor of chemistry at the Jardin des Plantes (the chief botanical garden in France), and member of the French Academy of Sciences (elected December 28, 1829 - Chemistry section). He was one of the first researchers to draw attention to the haloform reaction. In 1822, Serullas added potassium metal to a solution of iodine in ethanol and water to form potassium formate and iodoform, called in the language of that time ''hydroiodide of carbon'', and used as an antiseptic. He is buried in the Père Lachaise Cemetery Père Lachaise Cemetery (french: Cimetière du Père-Lachaise ; formerly , "East Cemetery") is the largest cemetery in Paris, France (). With more than 3.5 million visitors annually, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally emitted by rice plantations in small amounts. It is also produced in vast quantities estimated to be greater than 214,000 tons annually by algae and kelp in the world's temperate oceans, and in lesser amounts on land by terrestrial fungi and bacteria. It is used in organic synthesis as a source of methyl groups. Preparation and handling Iodomethane is formed via the exothermic reaction that occurs when iodine is added to a mixture of methanol with red phosphorus. The iodinating reagent is phosphorus triiodide that is formed ''in situ:'' :3 CH3OH + PI3 → 3 CH3I + H2PO3H Alternatively, it is prepared from the reaction of dimethyl sulfate with potassium iodide in the presence of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diiodomethane
Diiodomethane or methylene iodide, commonly abbreviated "MI", is an organoiodine compound. Diiodomethane is a colorless liquid; however, it decomposes upon exposure to light liberating iodine, which colours samples brownish. It is slightly soluble in water, but soluble in organic solvents. It has a relatively high refractive index of 1.741, and a surface tension of 0.0508 N·m−1.Website of Krüss'' (8.10.2009) Uses Because of its high density, diiodomethane is used in the determination of the density of mineral and other solid samples. It can also be used as an optical contact liquid, in conjunction with the gemmological refractometer, for determining the refractive index of certain gemstones. Diiodomethane is a reagent for installing the CH2 group. In the Simmons–Smith reaction, it is a source of methylene. In fact the Simmons–Smith reaction does not produce free carbene but proceeds via Zn-CH2I intermediates. Diiodomethane is also a source of the equivalent of CH22+. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Tetraiodide
Carbon tetraiodide is a tetrahalomethane with the molecular formula CI4. Being bright red, it is a relatively rare example of a highly colored methane derivative. It is only 2.3% by weight carbon, although other methane derivatives are known with still less carbon. Structure The tetrahedral molecule features C-I distances of 2.12 ± 0.02 Å. The molecule is slightly crowded with short contacts between iodine atoms of 3.459 ± 0.03 Å, and possibly for this reason, it is thermally and photochemically unstable. Carbon tetraiodide crystallizes in tetragonal crystal structure (''a'' 6.409, ''c'' 9.558 (.10−1 nm)). It has zero dipole moment due to its symmetrically substituted tetrahedral geometry. Properties, synthesis, uses Carbon tetraiodide is slightly reactive towards water, giving iodoform and I2. It is soluble in nonpolar organic solvents. It decomposes thermally and photochemically to tetraiodoethylene, C2I4. Its synthesis entails AlCl3-catalyzed halide exchange, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body. The International Agency for Research on Cancer (IARC) has listed acetaldehyde as a Group 1 carcinogen. Acetaldehyde is "one of the mos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
John Thomas Cooper
John Thomas Cooper (1790–1854) was an English chemist notable as a lecturer, chemical supplier and chemical analyst, at a time when interest was burgeoning in chemistry as a discipline of study and application. Biography Cooper was born in Greenwich and studied and for a short while practised medicine. Finding the life of general practitioner stressful and tiring, he turned instead to chemistry, to which he applied himself with zeal. Until 1842 he lectured in chemistry at a number of establishments, including the Russell Institution, the Aldersgate School of Medicine, and the Webb Street School of Anatomy and Medicine in Southwark. Cooper acted as a manufacturer and supplier of chemicals - "at one time the sole supplier of iodine in Britain" according to the Oxford Dictionary of National Biography. He devised or collaborated to produce a number of tools and techniques for which he won repute, including a hydrometer, an oxy-hydrogen microscope (the gasses providing a bright ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
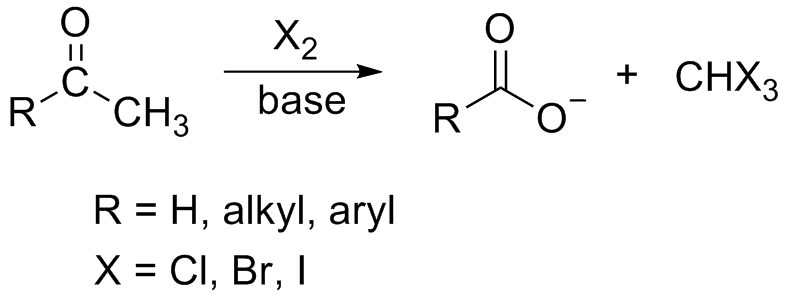
Haloform Reaction
In chemistry, the haloform reaction is a chemical reaction in which a haloform (, where X is a halogen) is produced by the exhaustive halogenation of an acetyl group (, where R can be either a hydrogen atom, an alkyl or an aryl group), in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups () or to produce chloroform (), bromoform (), or iodoform (). Note that fluoroform () can't be prepared in this way. Mechanism In the first step, the halogen dis-proportionates in the presence of hydroxide to give the halide and hypohalite. :Br2 + 2 OH- -> Br- + BrO- + H2O If a secondary alcohol is present, it is oxidized to a ketone by the hypohalite: If a methyl ketone is present, it reacts with the hypohalite in a three-step process: 1. Under basic conditions, the ketone undergoes keto-enol tautomerisation. The enolate undergoes electrophilic attack by the hypohalite (containing a halogen with a formal +1 charge). : 2. When the α(al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society (professional association) in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 34,000 in the UK and a further 8,000 abroad. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |