|
Jocic–Reeve Reaction
In organic chemistry, the Jocic reaction, also called the Jocic–Reeve reaction (named after Zivojin Jocic and Wilkins Reeve) is a name reaction that generates α-substituted carboxylic acids from trichloromethylcarbinols and corresponding nucleophiles in the presence of sodium hydroxide. The reaction involves nucleophilic displacement of the hydroxyl group in a 1,1,1-trichloro-2-hydroxyalkyl structure with concomitant conversion of the trichloromethyl portion to a carboxylic acid or similar functional group. Mechanism The reaction mechanism involves an epoxide intermediate that undergoes an SN2 reaction, SN2 reaction by the nucleophile. As a result of this mechanistic aspect, the reaction can easily occur on secondary carbon, secondary or tertiary carbon, tertiary positions, and chiral products can be made by using chiral alcohol (chemistry), alcohol substrates. The reaction is one stage of the Corey–Link reaction, the Bargellini reaction, and other processes for synthesizing Π... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object. An object or a system is ''chiral'' if it is distinguishable from its mirror image; that is, it cannot be superimposed onto it. Conversely, a mirror image of an ''achiral'' object, such as a sphere, cannot be distinguished from the object. A chiral object and its mirror image are called ''enantiomorphs'' (Greek, "opposite forms") or, when referring to molecules, '' enantiomers''. A non-chiral object is called ''achiral'' (sometimes also ''amphichiral'') and can be superposed on its mirror image. The term was first used by Lord Kelvin in 1893 in the second Robert Boyle Lecture at the Oxford University Junior Scientific Club which was published in 1894: Human hands are perhaps the most recognized example of chirality. The left hand is a non-superimposable mirror image of the right hand; no matter ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula Na BH4. This white solid, usually encountered as an aqueous basic solution, is a reducing agent that finds application in papermaking and dye industries. It is also used as a reagent in organic synthesis. The compound was discovered in the 1940s by H. I. Schlesinger, who led a team seeking volatile uranium compounds.Hermann I Schlesinger and Herbert C Brown (1945)Preparation of alkali metal compounds. US Patent 2461661. Granted on 1949-02-15; expired on 1966-02-15. Results of this wartime research were declassified and published in 1953. Properties The compound is soluble in alcohols, certain ethers, and water, although it slowly hydrolyzes. Sodium borohydride is an odorless white to gray-white microcrystalline powder that often forms lumps. It can be purified by recrystallization from warm (50 °C) diglyme. Sodium borohydride is soluble ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethoxyethane
Dimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a colorless, aprotic, and liquid ether that is used as a solvent, especially in batteries. Dimethoxyethane is miscible with water. Production Monoglyme is produced industrially by the reaction of dimethylether with ethylene oxide: :CH3OCH3 + CH2CH2O ‚Üí CH3OCH2CH2OCH3 Applications as solvent and ligand left, 144px, Structure of the coordination complex NbCl3(dimethoxyethane)(3-hexyne).{{cite journal , doi=10.1021/ja8100837, title=New Tantalum Ligand-Free Catalyst System for Highly Selective Trimerization of Ethylene Affording 1-Hexene: New Evidence of a Metallacycle Mechanism, year=2009, last1=Arteaga-M√ºller, first1=Roc√≠o, last2=Tsurugi, first2=Hayato, last3=Saito, first3=Teruhiko, last4=Yanagawa, first4=Masao, last5=Oda, first5=Seiji, last6=Mashima, first6=Kazushi, journal=Journal of the American Chemical Society, volume=131, issue=15, pages=5370‚ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Alcohol
A primary alcohol is an alcohol in which the hydroxy group is bonded to a primary carbon atom. It can also be defined as a molecule containing a “–CH2OH” group. In contrast, a secondary alcohol has a formula “–CHROH” and a tertiary alcohol has a formula “–CR2OH”, where “R” indicates a carbon-containing group. Examples of primary alcohols include ethanol and 1-butanol. Methanol is also generally regarded as a primary alcohol, including the 1911 edition of the Encyclopædia Britannica,. See also * Alcohol (especially Nomenclature section for discussion on Secondary and Tertiary alcohols.) * Oxidation of primary alcohols to carboxylic acids The oxidation of primary alcohols to carboxylic acids is an important oxidation reaction in organic chemistry. When a primary alcohol is converted to a carboxylic acid, the terminal carbon atom increases its oxidation state by four. Oxidants able ... References {{organic-chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homologation Reaction
In organic chemistry, a homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A homologous series is a group of compounds that differ by a constant unit, generally a methylene () group. The reactants undergo a homologation when the number of a repeated structural unit in the molecules is increased. The most common homologation reactions increase the number of methylene () units in saturated chain within the molecule. For example, the reaction of aldehydes or ketones with diazomethane or methoxymethylenetriphenylphosphine to give the next homologue in the series. Examples of homologation reactions include: * Kiliani-Fischer synthesis, where an aldose molecule is elongated through a three-step process consisting of: *# Nucleophillic addition of cyanide to the carbonyl to form a cyanohydrin *# Hydrolysis to form a lactone *# Reduction to form the homologous aldose * Wittig reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form binary compounds with hydrogen, the exceptions being He, Ne, Ar, Kr, Pm, Os, Ir, Rn, Fr, and Ra. Exotic molecules such as positronium hydride have also been made. Bonds Bonds between hydrogen and the other elements range from highly to somewhat covalent. Some hydrides, e.g. boron hydrides, do not conform to classical electron-counting rules and the bonding is described in terms of multi-centered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
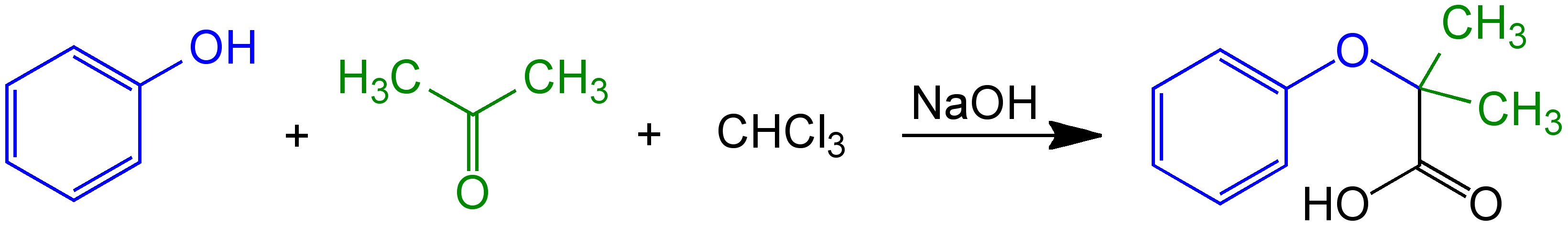
Bargellini Reaction
The Bargellini reaction is a chemical reaction discovered in 1906 by Italian chemist Guido Bargellini. The original reaction was a mixture of the reagents phenol, chloroform, and acetone in the presence of a sodium hydroxide solution. Prior to Bargellini's research, the product attributed to this multi-component reaction (MCR) had been described as a phenol derivative in chemistry texts at the time. However, Bargellini demonstrated that a carboxylic acid derivative was actually the correct structure. Later, organic chemists have used the reaction as a general method of organic synthesis for highly hindered or bulky morpholinones or piperazinones from ketones (particularly acetone) and either β-amino alcohols or diamines. History Guido Bargellini was a disciple of Hermann Emil Louis Fischer, the German chemist and Nobel laureate famous for the eponymous Fischer esterification reaction. Bargellini did his post-doctoral lab research in Fischer's laboratory. He spent mos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corey–Link Reaction
In organic chemistry, the Corey–Link reaction is a name reaction that converts a 1,1,1-trichloro-2-keto structure into a 2-aminocarboxylic acid (an alpha amino acid) or other acyl functional group with control of the chirality at the alpha position. The reaction is named for E.J. Corey and John Link, who first reported the reaction sequence. Process The first stage of the process is the reduction of the carbonyl to give a 1,1,1-trichloro-2-hydroxy structure. The original protocol used catecholborane with a small amount of one enantiomer of CBS catalyst (a Corey–Itsuno reduction). The choice of chirality of the catalyst thus gives selectivity for one or the other stereochemistry of the alcohol, which subsequently controls the stereochemistry of the amino substituent in the ultimate product. This 2-hydroxy structure then undergoes a Jocic–Reeve reaction using azide as the nucleophile. The multistep reaction mechanism begins with deprotonation of the alcohol, followed by the o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |