|
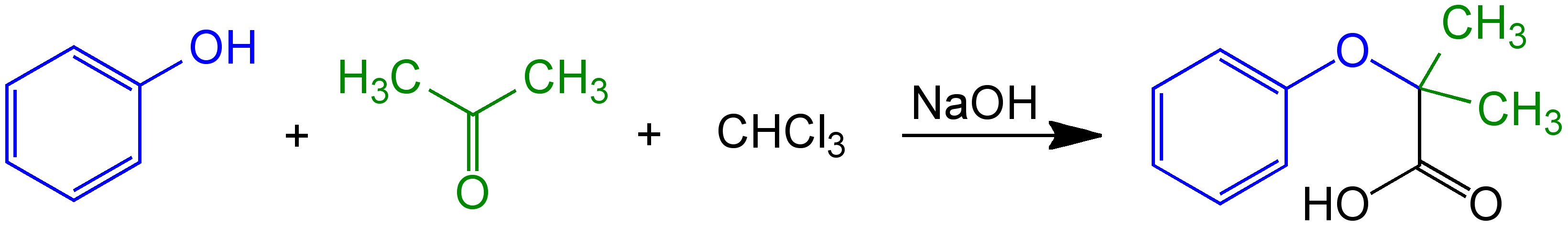
Bargellini Reaction
The Bargellini reaction is a chemical reaction discovered in 1906 by Italian chemist Guido Bargellini. The original reaction was a mixture of the reagents phenol, chloroform, and acetone in the presence of a sodium hydroxide solution. Prior to Bargellini's research, the product attributed to this multi-component reaction (MCR) had been described as a phenol derivative in chemistry texts at the time. However, Bargellini demonstrated that a carboxylic acid derivative was actually the correct structure. Later, organic chemists have used the reaction as a general method of organic synthesis for highly hindered or bulky morpholinones or piperazinones from ketones (particularly acetone) and either β-amino alcohols or diamines. History Guido Bargellini was a disciple of Hermann Emil Louis Fischer, the German chemist and Nobel laureate famous for the eponymous Fischer esterification reaction. Bargellini did his post-doctoral lab research in Fischer's laboratory. He spent mos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guido Bargellini
Guido Bargellini (1879–1963) was an Italian organic chemist. He specialized in natural product chemistry, in particular, flavonoid dyes and coumarins, and the compound santonin. He was admitted to the Accademia dei Lincei in 1946. The Bargellini reaction The Bargellini reaction is a chemical reaction discovered in 1906 by Italian chemist Guido Bargellini. The original reaction was a mixture of the reagents phenol, chloroform, and acetone in the presence of a sodium hydroxide solution. Prior to B ... is named for him. References Eintrag bei treccani.it 1879 births 1963 deaths Italian chemists Organic chemists {{Italy-scientist-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemisches Zentralblatt
''Chemisches Zentralblatt'' is the first and oldest abstracts journal published in the field of chemistry. It covers the chemical literature from 1830 to 1969 and describes therefore the "birth" of chemistry as science, in contrast to alchemy. The information contained in this German journal is comparable with the content of the leading source of chemical information Chemical Abstracts Service (CAS), which started publishing abstracts in English in 1907. ''Chemisches Zentralblatt'' was founded as ''Pharmaceutisches Centralblatt'' by Gustav Theodor FechnerM. Pflücke:'' Das Chemische Zentralblatt 125 Jahre alt'', Angew. Chem. ''66'' (1954) 537-541, DOI: :10.1002/ange.19540661708. and published by Leopold Voß in LeipzigR. Willstätter: ''Zur Hundertjahrfeier des Chemischen Zentralblattes'', Angew. Chem. ''42'' (1925) 1049-1052, DOI: :10.1002/ange.19290424502. in 1830. In the first year, 544 pages containing 400 abstracts were published, reporting all relevant research results in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Tetrabromide
Tetrabromomethane, CBr4, also known as carbon tetrabromide, is a carbon bromide. Both names are acceptable under IUPAC nomenclature. Physical properties Tetrabromomethane has two polymorphs: crystalline II or β below 46.9 °C (320.0 K) and crystalline I or α above 46.9 °C. Monoclinic polymorph has space group ''C2/c'' with lattice constants: ''a'' = 20.9, ''b'' = 12.1, ''c'' = 21.2 (.10−1 nm), β = 110.5°.F. Brezina, J. Mollin, R. Pastorek, Z. Sindelar. ''Chemicke tabulky anorganickych sloucenin'' (''Chemical tables of inorganic compounds''). SNTL, 1986. Bond energy of C-Br is 235 kJ.mol−1.N. N. Greenwood, A. Earnshaw. ''Chemie prvku'' (''Chemistry of the Elements''). Informatorium, Prague, 1993. Due to its symmetrically substituted tetrahedral structure, its dipole moment is 0 Debye. Critical temperature is 439 °C (712 K) and critical pressure is 4.26 MPa. Plastic crystallinity The high temperature α phase is known as a plastic crystal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Tetrachloride
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemical formula CCl4. It is a colourless liquid with a "sweet" smell that can be detected at low levels. It is practically incombustible at lower temperatures. It was formerly widely used in fire extinguishers, as a precursor to refrigerants and as a cleaning agent, but has since been phased out because of environmental and safety concerns. Exposure to high concentrations of carbon tetrachloride (including vapor) can affect the central nervous system and degenerate the liver and kidneys. Prolonged exposure can be fatal. Properties In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedron, tetrahedral configuration joined to a central carbon atom by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloral
Chloral, also known as trichloroacetaldehyde or trichloroethanal, is the organic compound with the formula Cl3CCHO. This aldehyde is a colourless oily liquid that is soluble in a wide range of solvents. It reacts with water to form chloral hydrate, a once widely used sedative and hypnotic substance. Production Chloral was first prepared, and named, by the German chemist Justus von Liebig in 1832. Liebig treated anhydrous ethanol with dry chlorine gas. Chloral is produced commercially by the chlorination of acetaldehyde in the presence of hydrochloric acid, producing chloral hydrate. Ethanol can also be used as a feedstock. This reaction is catalyzed by antimony trichloride: :H3CCHO + 3 Cl2 + H2O ‚Üí Cl3CCH(OH)2 + 3 HCl The chloral hydrate is distilled from the reaction mixture. The distillate is then dehydrated with concentrated sulfuric acid, after which the heavier acid layer (containing the water) is drawn off: :Cl3CCH(OH)2 ‚Üí Cl3CCHO + H2O The resulting product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromoform
Bromoform (CHBr3) is a brominated organic solvent, colorless liquid at room temperature, with a high refractive index, very high density, and sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. Bromoform can be prepared by the haloform reaction using acetone and sodium hypobromite, by the electrolysis of potassium bromide in ethanol, or by treating chloroform with aluminium bromide. Currently its main use is as a laboratory reagent. Structure The molecule adopts tetrahedral molecular geometry with C3v symmetry. Uses Only small quantities of bromoform are currently produced industrially in the United States. In the past, it was used as a solvent, sedative and flame retardant, but now it is mainly used as a laboratory reagent, for example as an extraction solvent. Bromoform also has medical uses; injections of bromoform are sometimes used instead of epinephrine to treat severe asthma cases. Bromo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corey–Link Reaction
In organic chemistry, the Corey–Link reaction is a name reaction that converts a 1,1,1-trichloro-2-keto structure into a 2-aminocarboxylic acid (an alpha amino acid) or other acyl functional group with control of the chirality at the alpha position. The reaction is named for E.J. Corey and John Link, who first reported the reaction sequence. Process The first stage of the process is the reduction of the carbonyl to give a 1,1,1-trichloro-2-hydroxy structure. The original protocol used catecholborane with a small amount of one enantiomer of CBS catalyst (a Corey–Itsuno reduction). The choice of chirality of the catalyst thus gives selectivity for one or the other stereochemistry of the alcohol, which subsequently controls the stereochemistry of the amino substituent in the ultimate product. This 2-hydroxy structure then undergoes a Jocic–Reeve reaction using azide as the nucleophile. The multistep reaction mechanism begins with deprotonation of the alcohol, followed by the o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jocic–Reeve Reaction
In organic chemistry, the Jocic reaction, also called the Jocic–Reeve reaction (named after Zivojin Jocic and Wilkins Reeve) is a name reaction that generates α-substituted carboxylic acids from trichloromethylcarbinols and corresponding nucleophiles in the presence of sodium hydroxide. The reaction involves nucleophilic displacement of the hydroxyl group in a 1,1,1-trichloro-2-hydroxyalkyl structure with concomitant conversion of the trichloromethyl portion to a carboxylic acid or similar functional group. Mechanism The reaction mechanism involves an epoxide intermediate that undergoes an SN2 reaction, SN2 reaction by the nucleophile. As a result of this mechanistic aspect, the reaction can easily occur on secondary carbon, secondary or tertiary carbon, tertiary positions, and chiral products can be made by using chiral alcohol (chemistry), alcohol substrates. The reaction is one stage of the Corey–Link reaction, the Bargellini reaction, and other processes for synthesizing Π... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |