Americium dioxide on:
[Wikipedia]
[Google]
[Amazon]
Americium is a synthetic radioactive chemical element with the
 Although americium was likely produced in previous nuclear experiments, it was first intentionally synthesized, isolated and identified in late autumn 1944, at the University of California, Berkeley, by Glenn T. Seaborg, Leon O. Morgan,
Although americium was likely produced in previous nuclear experiments, it was first intentionally synthesized, isolated and identified in late autumn 1944, at the University of California, Berkeley, by Glenn T. Seaborg, Leon O. Morgan,
 The longest-lived and most common isotopes of americium, 241Am and 243Am, have half-lives of 432.2 and 7,370 years, respectively. Therefore, any
The longest-lived and most common isotopes of americium, 241Am and 243Am, have half-lives of 432.2 and 7,370 years, respectively. Therefore, any
, Los Alamos National Laboratory, Retrieved 28 November 2010 Americium is produced mostly artificially in small quantities, for research purposes. A tonne of spent nuclear fuel contains about 100 grams of various americium isotopes, mostly 241Am and 243Am. Their prolonged radioactivity is undesirable for the disposal, and therefore americium, together with other long-lived actinides, must be neutralized. The associated procedure may involve several steps, where americium is first separated and then converted by neutron bombardment in special reactors to short-lived nuclides. This procedure is well known as
 Americium has been produced in small quantities in nuclear reactors for decades, and kilograms of its 241Am and 243Am isotopes have been accumulated by now.Greenwood, p. 1262 Nevertheless, since it was first offered for sale in 1962, its price, about of 241Am, remains almost unchanged owing to the very complex separation procedure.Smoke detectors and americium
Americium has been produced in small quantities in nuclear reactors for decades, and kilograms of its 241Am and 243Am isotopes have been accumulated by now.Greenwood, p. 1262 Nevertheless, since it was first offered for sale in 1962, its price, about of 241Am, remains almost unchanged owing to the very complex separation procedure.Smoke detectors and americium
, World Nuclear Association, January 2009, Retrieved 28 November 2010 The heavier isotope 243Am is produced in much smaller amounts; it is thus more difficult to separate, resulting in a higher cost of the order .Hammond C. R. "The elements" in Americium is not synthesized directly from uranium – the most common reactor material – but from the plutonium isotope 239Pu. The latter needs to be produced first, according to the following nuclear process: :^_U -> ce^_U -> beta^-23.5 \ \ce] ^_Np -> beta^-2.3565 \ \ce] ^_Pu
The capture of two neutrons by 239Pu (a so-called (n,γ) reaction), followed by a β-decay, results in 241Am:
: ^_Pu -> ce^_Pu -> beta^-14.35 \ \ce] ^_Am
The plutonium present in spent nuclear fuel contains about 12% of 241Pu. Because it ^_Pu -> ce\ ^_Pu -> beta^-4.956 \ \ce] ^_Am
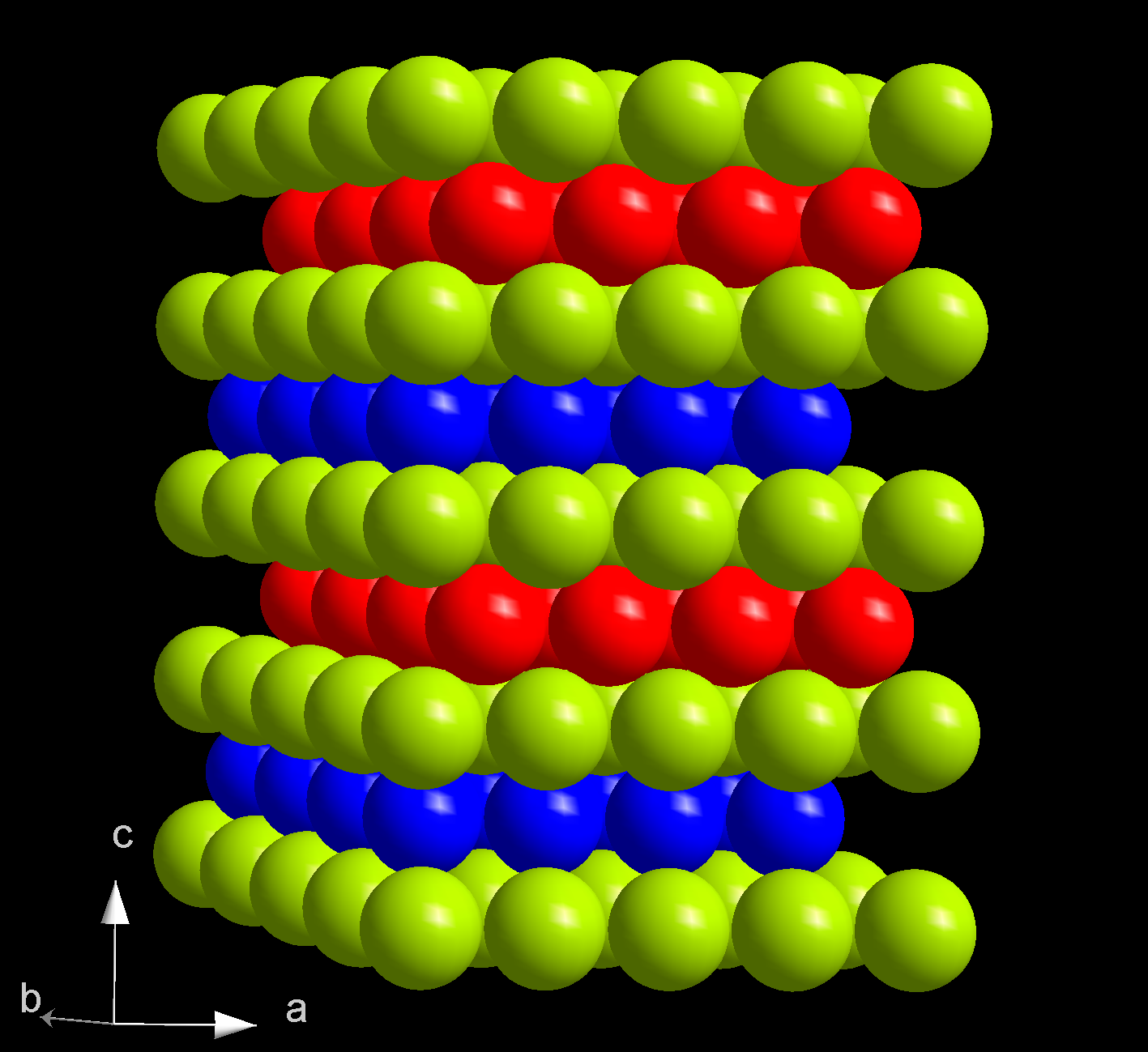 In the
In the
+ \underset -> \atop 400 - 500 ^\circ \ce C +
Americium(III) fluoride (AmF3) is poorly soluble and precipitates upon reaction of Am3+ and fluoride ions in weak acidic solutions:
: Am^3+ + 3F^- -> AmF3(v)
The tetravalent americium(IV) fluoride (AmF4) is obtained by reacting solid americium(III) fluoride with molecular 2AmF3 + F2 -> 2AmF4
Another known form of solid tetravalent americium fluoride is KAmF5.Penneman, p. 6 Tetravalent americium has also been observed in the aqueous phase. For this purpose, black Am(OH)4 was dissolved in 15- M NH4F with the americium concentration of 0.01 M. The resulting reddish solution had a characteristic optical absorption spectrum which is similar to that of AmF4 but differed from other oxidation states of americium. Heating the Am(IV) solution to 90 °C did not result in its disproportionation or reduction, however a slow reduction was observed to Am(III) and assigned to self-irradiation of americium by alpha particles.
Most americium(III) halides form hexagonal crystals with slight variation of the color and exact structure between the halogens. So, chloride (AmCl3) is reddish and has a structure isotypic to
 Analogous to uranocene, americium forms the organometallic compound amerocene with two cyclooctatetraene ligands, with the chemical formula (η8-C8H8)2Am. A cyclopentadienyl complex is also known that is likely to be stoichiometrically AmCp3.
Formation of the complexes of the type Am(n-C3H7-BTP)3, where BTP stands for 2,6-di(1,2,4-triazin-3-yl)pyridine, in solutions containing n-C3H7-BTP and Am3+ ions has been confirmed by EXAFS. Some of these BTP-type complexes selectively interact with americium and therefore are useful in its selective separation from lanthanides and another actinides.
Analogous to uranocene, americium forms the organometallic compound amerocene with two cyclooctatetraene ligands, with the chemical formula (η8-C8H8)2Am. A cyclopentadienyl complex is also known that is likely to be stoichiometrically AmCp3.
Formation of the complexes of the type Am(n-C3H7-BTP)3, where BTP stands for 2,6-di(1,2,4-triazin-3-yl)pyridine, in solutions containing n-C3H7-BTP and Am3+ ions has been confirmed by EXAFS. Some of these BTP-type complexes selectively interact with americium and therefore are useful in its selective separation from lanthanides and another actinides.
"Evaluation of nuclear criticality safety data and limits for actinides in transport"
p. 16. Scarcity and high price yet hinder application of americium as a
Performance of Home Smoke Alarms Analysis of the Response of Several Available Technologies in Residential Fire Settings
, NIST Technical Note 1455-1
, G.L. Kulcinski, NEEP 602 Course Notes (Spring 2000), Nuclear Power in Space, University of Wisconsin Fusion Technology Institute (see last page) Although americium produces less heat and electricity – the power yield is 114.7 mW/g for 241Am and 6.31 mW/g for 243Am (cf. 390 mW/g for 238Pu) – and its radiation poses more threat to humans owing to neutron emission, the
^_Am -> ^_Np + ^_He + \gamma
: ^_Be + ^_He -> ^_C + ^_n + \gamma
The most widespread use of 241AmBe neutron sources is a
^_Am -> ce^_Am -> beta^-10.1 \ \ce] ^_Cm
Irradiation of 241Am by 12C or 22Ne ions yields the isotopes 247Es ( einsteinium) or 260Db ( dubnium), respectively. Furthermore, the element berkelium (243Bk isotope) had been first intentionally produced and identified by bombarding 241Am with alpha particles, in 1949, by the same Berkeley group, using the same 60-inch cyclotron. Similarly, nobelium was produced at the Joint Institute for Nuclear Research, Dubna, Russia, in 1965 in several reactions, one of which included irradiation of 243Am with 15N ions. Besides, one of the synthesis reactions for lawrencium, discovered by scientists at Berkeley and Dubna, included bombardment of 243Am with 18O.
The Radioactive Boy Scout: When a teenager attempts to build a breeder reactor
''
The radiochemistry of americium and curium
University of California, Los Alamos, California, 1960 *
at '' The Periodic Table of Videos'' (University of Nottingham)
ATSDR – Public Health Statement: Americium
{{Authority control Chemical elements Chemical elements with double hexagonal close-packed structure Actinides Carcinogens Synthetic elements
symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
located under the lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttr ...
element europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lanth ...
, and thus by analogy was named after the Americas
The Americas, which are sometimes collectively called America, are a landmass comprising the totality of North and South America. The Americas make up most of the land in Earth's Western Hemisphere and comprise the New World.
Along with th ...
.
Americium was first produced in 1944 by the group of Glenn T. Seaborg from Berkeley, California, at the Metallurgical Laboratory
The Metallurgical Laboratory (or Met Lab) was a scientific laboratory at the University of Chicago that was established in February 1942 to study and use the newly discovered chemical element plutonium. It researched plutonium's chemistry and m ...
of the University of Chicago, as part of the Manhattan Project. Although it is the third element in the transuranic series, it was discovered fourth, after the heavier curium
Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first inte ...
. The discovery was kept secret and only released to the public in November 1945. Most americium is produced by uranium or plutonium being bombarded with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains about 100 grams of americium. It is widely used in commercial ionization chamber smoke detectors, as well as in neutron sources and industrial gauges. Several unusual applications, such as nuclear batteries or fuel for space ships with nuclear propulsion, have been proposed for the isotope 242mAm, but they are as yet hindered by the scarcity and high price of this nuclear isomer.
Americium is a relatively soft radioactive metal with silvery appearance. Its most common isotopes are 241Am and 243Am. In chemical compounds, americium usually assumes the oxidation state +3, especially in solutions. Several other oxidation states are known, ranging from +2 to +7, and can be identified by their characteristic optical absorption spectra. The crystal lattice of solid americium and its compounds contain small intrinsic radiogenic defects, due to metamictization
Metamictisation (sometimes called metamictization or metamiction) is a natural process resulting in the gradual and ultimately complete destruction of a mineral's crystal structure, leaving the mineral amorphous. The affected material is therefore ...
induced by self-irradiation with alpha particles, which accumulates with time; this can cause a drift of some material properties over time, more noticeable in older samples.
History
 Although americium was likely produced in previous nuclear experiments, it was first intentionally synthesized, isolated and identified in late autumn 1944, at the University of California, Berkeley, by Glenn T. Seaborg, Leon O. Morgan,
Although americium was likely produced in previous nuclear experiments, it was first intentionally synthesized, isolated and identified in late autumn 1944, at the University of California, Berkeley, by Glenn T. Seaborg, Leon O. Morgan, Ralph A. James
Ralph Arthur James (23 September 1920 in Salt Lake City, Utah – 24 February 1973 in Alamo, California) was an American chemist at the University of Chicago who co-discovered the elements curium (1944) and americium (1944–1945). Later ...
, and Albert Ghiorso. They used a 60-inch cyclotron at the University of California, Berkeley. The element was chemically identified at the Metallurgical Laboratory (now Argonne National Laboratory
Argonne National Laboratory is a science and engineering research United States Department of Energy National Labs, national laboratory operated by University of Chicago, UChicago Argonne LLC for the United States Department of Energy. The facil ...
) of the University of Chicago. Following the lighter neptunium, plutonium, and heavier curium
Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first inte ...
, americium was the fourth transuranium element to be discovered. At the time, the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
had been restructured by Seaborg to its present layout, containing the actinide row below the lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttr ...
one. This led to americium being located right below its twin lanthanide element europium; it was thus by analogy named after the Americas
The Americas, which are sometimes collectively called America, are a landmass comprising the totality of North and South America. The Americas make up most of the land in Earth's Western Hemisphere and comprise the New World.
Along with th ...
: "The name americium (after the Americas) and the symbol Am are suggested for the element on the basis of its position as the sixth member of the actinide rare-earth series, analogous to europium, Eu, of the lanthanide series."Greenwood, p. 1252
The new element was isolated from its oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
s in a complex, multi-step process. First plutonium-239 nitrate (239PuNO3) solution was coated on a platinum foil of about 0.5 cm2 area, the solution was evaporated and the residue was converted into plutonium dioxide (PuO2) by calcining. After cyclotron irradiation, the coating was dissolved with nitric acid, and then precipitated as the hydroxide using concentrated aqueous ammonia solution. The residue was dissolved in perchloric acid. Further separation was carried out by ion exchange, yielding a certain isotope of curium. The separation of curium and americium was so painstaking that those elements were initially called by the Berkeley group as ''pandemonium
Pandæmonium, Pandemonium or Pandamonium may refer to:
Literature
* Pandæmonium (''Paradise Lost''), capital of Hell in John Milton's epic poem ''Paradise Lost''
* ''Pandaemonium'' (history book), a book by Humphrey Jennings, published posthum ...
'' (from Greek for ''all demons'' or ''hell'') and ''delirium
Delirium (also known as acute confusional state) is an organically caused decline from a previous baseline of mental function that develops over a short period of time, typically hours to days. Delirium is a syndrome encompassing disturbances in ...
'' (from Latin for ''madness'').
Initial experiments yielded four americium isotopes: 241Am, 242Am, 239Am and 238Am. Americium-241 was directly obtained from plutonium upon absorption of two neutrons. It decays by emission of a α-particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produce ...
to 237Np; the half-life of this decay was first determined as years but then corrected to 432.2 years.
:
: The times are half-lives
The second isotope 242Am was produced upon neutron bombardment of the already-created 241Am. Upon rapid β-decay, 242Am converts into the isotope of curium 242Cm (which had been discovered previously). The half-life of this decay was initially determined at 17 hours, which was close to the presently accepted value of 16.02 h.
:
The discovery of americium and curium in 1944 was closely related to the Manhattan Project; the results were confidential and declassified only in 1945. Seaborg leaked the synthesis of the elements 95 and 96 on the U.S. radio show for children '' Quiz Kids'' five days before the official presentation at an American Chemical Society meeting on 11 November 1945, when one of the listeners asked whether any new transuranium element besides plutonium and neptunium had been discovered during the war. After the discovery of americium isotopes 241Am and 242Am, their production and compounds were patented listing only Seaborg as the inventor. The initial americium samples weighed a few micrograms; they were barely visible and were identified by their radioactivity. The first substantial amounts of metallic americium weighing 40–200 micrograms were not prepared until 1951 by reduction of americium(III) fluoride with barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
metal in high vacuum at 1100 °C.
Occurrence
 The longest-lived and most common isotopes of americium, 241Am and 243Am, have half-lives of 432.2 and 7,370 years, respectively. Therefore, any
The longest-lived and most common isotopes of americium, 241Am and 243Am, have half-lives of 432.2 and 7,370 years, respectively. Therefore, any primordial
Primordial may refer to:
* Primordial era, an era after the Big Bang. See Chronology of the universe
* Primordial sea (a.k.a. primordial ocean, ooze or soup). See Abiogenesis
* Primordial nuclide, nuclides, a few radioactive, that formed before ...
americium (americium that was present on Earth during its formation) should have decayed by now. Trace amounts of americium probably occur naturally in uranium minerals as a result of nuclear reactions, though this has not been confirmed.
Existing americium is concentrated in the areas used for the atmospheric nuclear weapons tests conducted between 1945 and 1980, as well as at the sites of nuclear incidents, such as the Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is one of only two nuc ...
. For example, the analysis of the debris at the testing site of the first U.S. hydrogen bomb
A thermonuclear weapon, fusion weapon or hydrogen bomb (H bomb) is a second-generation nuclear weapon design. Its greater sophistication affords it vastly greater destructive power than first-generation nuclear bombs, a more compact size, a lowe ...
, Ivy Mike, (1 November 1952, Enewetak Atoll
Enewetak Atoll (; also spelled Eniwetok Atoll or sometimes Eniewetok; mh, Ānewetak, , or , ; known to the Japanese as Brown Atoll or Brown Island; ja, ブラウン環礁) is a large coral atoll of 40 islands in the Pacific Ocean and with it ...
), revealed high concentrations of various actinides including americium; but due to military secrecy, this result was not published until later, in 1956. Trinitite, the glassy residue left on the desert floor near Alamogordo, New Mexico, after the plutonium-based Trinity nuclear bomb test on 16 July 1945, contains traces of americium-241. Elevated levels of americium were also detected at the crash site of a US Boeing B-52 bomber aircraft, which carried four hydrogen bombs, in 1968 in Greenland.
In other regions, the average radioactivity of surface soil due to residual americium is only about 0.01 picocuries/g (0.37 mBq/g). Atmospheric americium compounds are poorly soluble in common solvents and mostly adhere to soil particles. Soil analysis revealed about 1,900 times higher concentration of americium inside sandy soil particles than in the water present in the soil pores; an even higher ratio was measured in loam
Loam (in geology and soil science) is soil composed mostly of sand (particle size > ), silt (particle size > ), and a smaller amount of clay (particle size < ). By weight, its mineral composition is about 40–40–20% concentration of sand–sil ...
soils.Human Health Fact Sheet on Americium, Los Alamos National Laboratory, Retrieved 28 November 2010 Americium is produced mostly artificially in small quantities, for research purposes. A tonne of spent nuclear fuel contains about 100 grams of various americium isotopes, mostly 241Am and 243Am. Their prolonged radioactivity is undesirable for the disposal, and therefore americium, together with other long-lived actinides, must be neutralized. The associated procedure may involve several steps, where americium is first separated and then converted by neutron bombardment in special reactors to short-lived nuclides. This procedure is well known as
nuclear transmutation
Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.
A transmutatio ...
, but it is still being developed for americium. The transuranic elements from americium to fermium occurred naturally in the natural nuclear fission reactor at Oklo, but no longer do so.
Americium is also one of the elements that have been detected in Przybylski's Star.
Synthesis and extraction
Isotope nucleosynthesis
 Americium has been produced in small quantities in nuclear reactors for decades, and kilograms of its 241Am and 243Am isotopes have been accumulated by now.Greenwood, p. 1262 Nevertheless, since it was first offered for sale in 1962, its price, about of 241Am, remains almost unchanged owing to the very complex separation procedure.Smoke detectors and americium
Americium has been produced in small quantities in nuclear reactors for decades, and kilograms of its 241Am and 243Am isotopes have been accumulated by now.Greenwood, p. 1262 Nevertheless, since it was first offered for sale in 1962, its price, about of 241Am, remains almost unchanged owing to the very complex separation procedure.Smoke detectors and americium, World Nuclear Association, January 2009, Retrieved 28 November 2010 The heavier isotope 243Am is produced in much smaller amounts; it is thus more difficult to separate, resulting in a higher cost of the order .Hammond C. R. "The elements" in Americium is not synthesized directly from uranium – the most common reactor material – but from the plutonium isotope 239Pu. The latter needs to be produced first, according to the following nuclear process: :
beta-decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
s to 241Am, 241Pu can be extracted and may be used to generate further 241Am. However, this process is rather slow: half of the original amount of 241Pu decays to 241Am after about 15 years, and the 241Am amount reaches a maximum after 70 years.
The obtained 241Am can be used for generating heavier americium isotopes by further neutron capture inside a nuclear reactor. In a light water reactor (LWR), 79% of 241Am converts to 242Am and 10% to its nuclear isomer 242mAm:The "metastable" state is marked by the letter m.
:
Americium-242
Americium (95Am) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no known stable isotopes. The first isotope to be synthesized was 241Am in 1944. The artificial element decays by e ...
has a half-life of only 16 hours, which makes its further conversion to 243Am extremely inefficient. The latter isotope is produced instead in a process where 239Pu captures four neutrons under high neutron flux:
: Metal generation
Most synthesis routines yield a mixture of different actinide isotopes in oxide forms, from which isotopes of americium can be separated. In a typical procedure, the spent reactor fuel (e.g. MOX fuel) is dissolved in nitric acid, and the bulk of uranium and plutonium is removed using a PUREX-type extraction (Plutonium–URanium EXtraction) with tributyl phosphate in a hydrocarbon. The lanthanides and remaining actinides are then separated from the aqueous residue ( raffinate) by adiamide
Diamide may refer to:
* Diamide, any chemical compound containing two amide groups
* Diamide, a synonym for tetramethylazodicarboxamide
* Diamide insecticides, a subclass of ryanoid insecticides
{{chemistry index ...
-based extraction, to give, after stripping, a mixture of trivalent actinides and lanthanides. Americium compounds are then selectively extracted using multi-step chromatographic and centrifugation techniques with an appropriate reagent. A large amount of work has been done on the solvent extraction of americium. For example, a 2003 EU-funded project codenamed "EUROPART" studied triazines and other compounds as potential extraction agents. A ''bis''-triazinyl bipyridine complex was proposed in 2009 as such a reagent is highly selective to americium (and curium). Separation of americium from the highly similar curium can be achieved by treating a slurry of their hydroxides in aqueous sodium bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
with ozone, at elevated temperatures. Both Am and Cm are mostly present in solutions in the +3 valence state; whereas curium remains unchanged, americium oxidizes to soluble Am(IV) complexes which can be washed away.
Metallic americium is obtained by reduction from its compounds. Americium(III) fluoride was first used for this purpose. The reaction was conducted using elemental barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
as reducing agent in a water- and oxygen-free environment inside an apparatus made of tantalum and tungsten.''Gmelin Handbook of Inorganic Chemistry'', System No. 71, transuranics, Part B 1, pp. 57–67.Penneman, p. 3
:
An alternative is the reduction of americium dioxide by metallic lanthanum or thorium:
:
Physical properties
 In the
In the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
, americium is located to the right of plutonium, to the left of curium, and below the lanthanide europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lanth ...
, with which it shares many physical and chemical properties. Americium is a highly radioactive element. When freshly prepared, it has a silvery-white metallic lustre, but then slowly tarnishes in air. With a density of 12 g/cm3, americium is less dense than both curium (13.52 g/cm3) and plutonium (19.8 g/cm3); but has a higher density than europium (5.264 g/cm3)—mostly because of its higher atomic mass. Americium is relatively soft and easily deformable and has a significantly lower bulk modulus than the actinides before it: Th, Pa, U, Np and Pu. Its melting point of 1173 °C is significantly higher than that of plutonium (639 °C) and europium (826 °C), but lower than for curium (1340 °C).
At ambient conditions, americium is present in its most stable α form which has a hexagonal crystal symmetry, and a space group P63/mmc with cell parameters ''a'' = 346.8 pm and ''c'' = 1124 pm, and four atoms per unit cell. The crystal consists of a double- hexagonal close packing with the layer sequence ABAC and so is isotypic with α-lanthanum and several actinides such as α-curium. The crystal structure of americium changes with pressure and temperature. When compressed at room temperature to 5 GPa, α-Am transforms to the β modification, which has a face-centered cubic (''fcc'') symmetry, space group Fmm and lattice constant ''a'' = 489 pm. This ''fcc'' structure is equivalent to the closest packing with the sequence ABC. Upon further compression to 23 GPa, americium transforms to an orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a r ...
γ-Am structure similar to that of α-uranium. There are no further transitions observed up to 52 GPa, except for an appearance of a monoclinic phase at pressures between 10 and 15 GPa. There is no consistency on the status of this phase in the literature, which also sometimes lists the α, β and γ phases as I, II and III. The β-γ transition is accompanied by a 6% decrease in the crystal volume; although theory also predicts a significant volume change for the α-β transition, it is not observed experimentally. The pressure of the α-β transition decreases with increasing temperature, and when α-americium is heated at ambient pressure, at 770 °C it changes into an ''fcc'' phase which is different from β-Am, and at 1075 °C it converts to a body-centered cubic structure. The pressure-temperature phase diagram of americium is thus rather similar to those of lanthanum, praseodymium and neodymium.
As with many other actinides, self-damage of the crystal structure due to alpha-particle irradiation is intrinsic to americium. It is especially noticeable at low temperatures, where the mobility of the produced structure defects is relatively low, by broadening of X-ray diffraction
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
peaks. This effect makes somewhat uncertain the temperature of americium and some of its properties, such as electrical resistivity. So for americium-241, the resistivity at 4.2 K increases with time from about 2 µOhm·cm to 10 µOhm·cm after 40 hours, and saturates at about 16 µOhm·cm after 140 hours. This effect is less pronounced at room temperature, due to annihilation of radiation defects; also heating to room temperature the sample which was kept for hours at low temperatures restores its resistivity. In fresh samples, the resistivity gradually increases with temperature from about 2 µOhm·cm at liquid helium to 69 µOhm·cm at room temperature; this behavior is similar to that of neptunium, uranium, thorium and protactinium, but is different from plutonium and curium which show a rapid rise up to 60 K followed by saturation. The room temperature value for americium is lower than that of neptunium, plutonium and curium, but higher than for uranium, thorium and protactinium.
Americium is paramagnetic in a wide temperature range, from that of liquid helium, to room temperature and above. This behavior is markedly different from that of its neighbor curium which exhibits antiferromagnetic transition at 52 K. The thermal expansion coefficient of americium is slightly anisotropic and amounts to along the shorter ''a'' axis and for the longer ''c'' hexagonal axis. The enthalpy of dissolution
In thermochemistry, the enthalpy of solution ( heat of solution or enthalpy of solvation) is the enthalpy change associated with the dissolution of a substance in a solvent at constant pressure resulting in infinite dilution.
The enthalpy of so ...
of americium metal in hydrochloric acid at standard conditions is , from which the standard enthalpy change of formation (Δf''H''°) of aqueous Am3+ ion is . The standard potential Am3+/Am0 is .
Chemical properties
Americium metal readily reacts with oxygen and dissolves in aqueousacid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
s. The most stable oxidation state for americium is +3,.Penneman, p. 4 The chemistry of americium(III) has many similarities to the chemistry of lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttr ...
(III) compounds. For example, trivalent americium forms insoluble fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typ ...
, oxalate, iodate, hydroxide, phosphate and other salts. Compounds of americium in oxidation states 2, 4, 5, 6 and 7 have also been studied. This is the widest range that has been observed with actinide elements. The color of americium compounds in aqueous solution is as follows: Am3+ (yellow-reddish), Am4+ (yellow-reddish), ; (yellow), (brown) and (dark green).Greenwood, p. 1265 The absorption spectra have sharp peaks, due to ''f''-''f'' transitions' in the visible and near-infrared regions. Typically, Am(III) has absorption maxima at ca. 504 and 811 nm, Am(V) at ca. 514 and 715 nm, and Am(VI) at ca. 666 and 992 nm.
Americium compounds with oxidation state +4 and higher are strong oxidizing agents, comparable in strength to the permanganate ion () in acidic solutions.Wiberg, p. 1956 Whereas the Am4+ ions are unstable in solutions and readily convert to Am3+, compounds such as americium dioxide (AmO2) and americium(IV) fluoride
Americium(IV) fluoride is the inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a ...
(AmF4) are stable in the solid state.
The pentavalent oxidation state of americium was first observed in 1951. In acidic aqueous solution the ion is unstable with respect to disproportionation.Greenwood, p. 1275 The reaction
:
is typical. The chemistry of Am(V) and Am(VI) is comparable to the chemistry of uranium in those oxidation states. In particular, compounds like and are comparable to uranates and the ion is comparable to the uranyl ion, . Such compounds can be prepared by oxidation of Am(III) in dilute nitric acid with ammonium persulfate. Other oxidising agents that have been used include silver(I) oxide, ozone and sodium persulfate.
Chemical compounds
Oxygen compounds
Three americium oxides are known, with the oxidation states +2 (AmO), +3 (Am2O3) and +4 (AmO2).Americium(II) oxide
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was named ...
was prepared in minute amounts and has not been characterized in detail. Americium(III) oxide is a red-brown solid with a melting point of 2205 °C.Wiberg, p. 1972 Americium(IV) oxide
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was named ...
is the main form of solid americium which is used in nearly all its applications. As most other actinide dioxides, it is a black solid with a cubic ( fluorite) crystal structure.Greenwood, p. 1267
The oxalate of americium(III), vacuum dried at room temperature, has the chemical formula Am2(C2O4)3·7H2O. Upon heating in vacuum, it loses water at 240 °C and starts decomposing into AmO2 at 300 °C, the decomposition completes at about 470 °C. The initial oxalate dissolves in nitric acid with the maximum solubility of 0.25 g/L.Penneman, p. 5
Halides
Halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
s of americium are known for the oxidation states +2, +3 and +4,Wiberg, p. 1969 where the +3 is most stable, especially in solutions.
Reduction of Am(III) compounds with sodium amalgam yields Am(II) salts – the black halides AmCl2, AmBr2 and AmI2. They are very sensitive to oxygen and oxidize in water, releasing hydrogen and converting back to the Am(III) state. Specific lattice constants are:
* Orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a r ...
AmCl2: ''a'' = , ''b'' = and ''c'' =
* Tetragonal AmBr2: ''a'' = and ''c'' = . They can also be prepared by reacting metallic americium with an appropriate mercury halide HgX2, where X = Cl, Br or I:Greenwood, p. 1272
: fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
:Greenwood, p. 1271
: uranium(III) chloride
Uranium(III) chloride, UCl3, is a water soluble salt of uranium. UCl3 is used mostly to reprocess spent nuclear fuel. Uranium(III) chloride is synthesized in various ways from uranium(IV) chloride; however, UCl3 is less stable than UCl4.
Prepar ...
(space group P63/m) and the melting point of 715 °C. The fluoride is isotypic to LaF3 (space group P63/mmc) and the iodide to BiI3 (space group R). The bromide is an exception with the orthorhombic PuBr3-type structure and space group Cmcm. Crystals of americium hexahydrate (AmCl3·6H2O) can be prepared by dissolving americium dioxide in hydrochloric acid and evaporating the liquid. Those crystals are hygroscopic and have yellow-reddish color and a monoclinic crystal structure.
Oxyhalides of americium in the form AmVIO2X2, AmVO2X, AmIVOX2 and AmIIIOX can be obtained by reacting the corresponding americium halide with oxygen or Sb2O3, and AmOCl can also be produced by vapor phase hydrolysis:
: AmCl3 + H2O -> AmOCl + 2HCl
Chalcogenides and pnictides
The known chalcogenides of americium include thesulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds lar ...
AmS2, selenides AmSe2 and Am3Se4, and tellurides Am2Te3 and AmTe2. The pnictides
A pnictogen ( or ; from grc, wikt:πνίγω, πνῑ́γω "to choke" and wikt:-gen#English, -gen, "generator") is any of the chemical elements in group (periodic table), group 15 of the periodic table. Group 15 is also known as the nitro ...
of americium (243Am) of the AmX type are known for the elements phosphorus, arsenic, antimony and bismuth. They crystallize in the rock-salt lattice.
Silicides and borides
Americium monosilicide (AmSi) and "disilicide" (nominally AmSix with: 1.87 < x < 2.0) were obtained by reduction of americium(III) fluoride with elementary silicon in vacuum at 1050 °C (AmSi) and 1150−1200 °C (AmSix). AmSi is a black solid isomorphic with LaSi, it has an orthorhombic crystal symmetry. AmSix has a bright silvery lustre and a tetragonal crystal lattice (space group ''I''41/amd), it is isomorphic with PuSi2 and ThSi2. Borides of americium include AmB4 and AmB6. The tetraboride can be obtained by heating an oxide or halide of americium with magnesium diboride in vacuum or inert atmosphere.Organoamericium compounds
 Analogous to uranocene, americium forms the organometallic compound amerocene with two cyclooctatetraene ligands, with the chemical formula (η8-C8H8)2Am. A cyclopentadienyl complex is also known that is likely to be stoichiometrically AmCp3.
Formation of the complexes of the type Am(n-C3H7-BTP)3, where BTP stands for 2,6-di(1,2,4-triazin-3-yl)pyridine, in solutions containing n-C3H7-BTP and Am3+ ions has been confirmed by EXAFS. Some of these BTP-type complexes selectively interact with americium and therefore are useful in its selective separation from lanthanides and another actinides.
Analogous to uranocene, americium forms the organometallic compound amerocene with two cyclooctatetraene ligands, with the chemical formula (η8-C8H8)2Am. A cyclopentadienyl complex is also known that is likely to be stoichiometrically AmCp3.
Formation of the complexes of the type Am(n-C3H7-BTP)3, where BTP stands for 2,6-di(1,2,4-triazin-3-yl)pyridine, in solutions containing n-C3H7-BTP and Am3+ ions has been confirmed by EXAFS. Some of these BTP-type complexes selectively interact with americium and therefore are useful in its selective separation from lanthanides and another actinides.
Biological aspects
Americium is an artificial element of recent origin, and thus does not have a biological requirement. It is harmful to life. It has been proposed to use bacteria for removal of americium and otherheavy metals
upright=1.2, Crystals of osmium, a heavy metal nearly twice as dense as lead">lead.html" ;"title="osmium, a heavy metal nearly twice as dense as lead">osmium, a heavy metal nearly twice as dense as lead
Heavy metals are generally defined as ...
from rivers and streams. Thus, Enterobacteriaceae
Enterobacteriaceae is a large family (biology), family of Gram-negative bacteria. It was first proposed by Rahn in 1936, and now includes over 30 genera and more than 100 species. Its classification above the level of family is still a subject ...
of the genus ''Citrobacter
''Citrobacter'' is a genus of Gram-negative coliform bacteria in the family Enterobacteriaceae.
The species ''C. amalonaticus'', ''C. koseri'', and ''C. freundii'' can use citrate as a sole carbon source. ''Citrobacter'' species are differentia ...
'' precipitate americium ions from aqueous solutions, binding them into a metal-phosphate complex at their cell walls. Several studies have been reported on the biosorption and bioaccumulation
Bioaccumulation is the gradual accumulation of substances, such as pesticides or other chemicals, in an organism. Bioaccumulation occurs when an organism absorbs a substance at a rate faster than that at which the substance is lost or eliminated ...
of americium by bacteria and fungi.
Fission
The isotope 242mAm (half-life 141 years) has the largest cross sections for absorption of thermal neutrons (5,700 barns),Pfennig, G.; Klewe-Nebenius, H and Seelmann Eggebert, W. (Eds.): Karlsruhenuclide
A nuclide (or nucleide, from nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by Truman ...
, 7 Edition 2006. that results in a small critical mass for a sustained nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nu ...
. The critical mass for a bare 242mAm sphere is about 9–14 kg (the uncertainty results from insufficient knowledge of its material properties). It can be lowered to 3–5 kg with a metal reflector and should become even smaller with a water reflector. Such small critical mass is favorable for portable nuclear weapons, but those based on 242mAm are not known yet, probably because of its scarcity and high price. The critical masses of two other readily available isotopes, 241Am and 243Am, are relatively high – 57.6 to 75.6 kg for 241Am and 209 kg for 243Am.Institut de Radioprotection et de Sûreté Nucléaire"Evaluation of nuclear criticality safety data and limits for actinides in transport"
p. 16. Scarcity and high price yet hinder application of americium as a
nuclear fuel
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Most nuclear fuels contain heavy fissile actinide elements that are capable of undergoing ...
in nuclear reactors.
There are proposals of very compact 10-kW high-flux reactors using as little as 20 grams of 242mAm. Such low-power reactors would be relatively safe to use as neutron sources for radiation therapy in hospitals.
Isotopes
About 19 isotopes and 8 nuclear isomers are known for americium. There are two long-lived alpha-emitters; 243Am has a half-life of 7,370 years and is the most stable isotope, and 241Am has a half-life of 432.2 years. The most stable nuclear isomer is 242m1Am; it has a long half-life of 141 years. The half-lives of other isotopes and isomers range from 0.64 microseconds for 245m1Am to 50.8 hours for 240Am. As with most other actinides, the isotopes of americium with odd number of neutrons have relatively high rate of nuclear fission and low critical mass. Americium-241 decays to 237Np emitting alpha particles of 5 different energies, mostly at 5.486 MeV (85.2%) and 5.443 MeV (12.8%). Because many of the resulting states are metastable, they also emit gamma rays with the discrete energies between 26.3 and 158.5 keV.Americium-242
Americium (95Am) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no known stable isotopes. The first isotope to be synthesized was 241Am in 1944. The artificial element decays by e ...
is a short-lived isotope with a half-life of 16.02 h. It mostly (82.7%) converts by β-decay to 242Cm, but also by electron capture to 242Pu (17.3%). Both 242Cm and 242Pu transform via nearly the same decay chain through 238Pu down to 234U.
Nearly all (99.541%) of 242m1Am decays by internal conversion to 242Am and the remaining 0.459% by α-decay to 238Np. The latter subsequently decays to 238Pu and then to 234U.
Americium-243 transforms by α-emission into 239Np, which converts by β-decay to 239Pu, and the 239Pu changes into 235U by emitting an α-particle.
Applications
Ionization-type smoke detector
Americium is used in the most common type of household smoke detector, which uses 241Am in the form of americium dioxide as its source ofionizing radiation
Ionizing radiation (or ionising radiation), including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel ...
. This isotope is preferred over 226 Ra because it emits 5 times more alpha particles and relatively little harmful gamma radiation.
The amount of americium in a typical new smoke detector is 1 microcurie (37 kBq
The becquerel (; symbol: Bq) is the unit of radioactivity in the International System of Units (SI). One becquerel is defined as the activity of a quantity of radioactive material in which one nucleus decays per second. For applications relatin ...
) or 0.29 microgram. This amount declines slowly as the americium decays into neptunium-237, a different transuranic element with a much longer half-life (about 2.14 million years). With its half-life of 432.2 years, the americium in a smoke detector includes about 3% neptunium after 19 years, and about 5% after 32 years. The radiation passes through an ionization chamber, an air-filled space between two electrodes, and permits a small, constant current between the electrodes. Any smoke that enters the chamber absorbs the alpha particles, which reduces the ionization and affects this current, triggering the alarm. Compared to the alternative optical smoke detector, the ionization smoke detector is cheaper and can detect particles which are too small to produce significant light scattering; however, it is more prone to false alarms
A false alarm, also called a nuisance alarm, is the deceptive or erroneous report of an emergency, causing unnecessary panic and/or bringing resources (such as emergency services) to a place where they are not needed. False alarms may occur with ...
.Bukowski, R. W. ''et al''. (2007Performance of Home Smoke Alarms Analysis of the Response of Several Available Technologies in Residential Fire Settings
, NIST Technical Note 1455-1
Radionuclide
As 241Am has a roughly similar half-life to 238Pu (432.2 years vs. 87 years), it has been proposed as an active element of radioisotope thermoelectric generators, for example in spacecraft.Basic elements of static RTGs, G.L. Kulcinski, NEEP 602 Course Notes (Spring 2000), Nuclear Power in Space, University of Wisconsin Fusion Technology Institute (see last page) Although americium produces less heat and electricity – the power yield is 114.7 mW/g for 241Am and 6.31 mW/g for 243Am (cf. 390 mW/g for 238Pu) – and its radiation poses more threat to humans owing to neutron emission, the
European Space Agency
, owners =
, headquarters = Paris, Île-de-France, France
, coordinates =
, spaceport = Guiana Space Centre
, seal = File:ESA emblem seal.png
, seal_size = 130px
, image = Views in the Main Control Room (1205 ...
is considering using americium for its space probes.
Another proposed space-related application of americium is a fuel for space ships with nuclear propulsion. It relies on the very high rate of nuclear fission of 242mAm, which can be maintained even in a micrometer-thick foil. Small thickness avoids the problem of self-absorption of emitted radiation. This problem is pertinent to uranium or plutonium rods, in which only surface layers provide alpha-particles. The fission products of 242mAm can either directly propel the spaceship or they can heat a thrusting gas. They can also transfer their energy to a fluid and generate electricity through a magnetohydrodynamic generator.
One more proposal which utilizes the high nuclear fission rate of 242mAm is a nuclear battery. Its design relies not on the energy of the emitted by americium alpha particles, but on their charge, that is the americium acts as the self-sustaining "cathode". A single 3.2 kg 242mAm charge of such battery could provide about 140 kW of power over a period of 80 days. Even with all the potential benefits, the current applications of 242mAm are as yet hindered by the scarcity and high price of this particular nuclear isomer.
In 2019, researchers at the UK National Nuclear Laboratory and the University of Leicester demonstrated the use of heat generated by americium to illuminate a small light bulb. This technology could lead to systems to power missions with durations up to 400 years into interstellar space, where solar panels do not function.
Neutron source
The oxide of 241Am pressed with beryllium is an efficient neutron source. Here americium acts as the alpha source, and beryllium produces neutrons owing to its large cross-section for the (α,n) nuclear reaction: :neutron probe
A neutron probe is a device used to measure the quantity of water present in soil.
A typical neutron probe contains a pellet of americium-241 and beryllium. The alpha particles emitted by the decay of the americium collide with the light beryllium ...
– a device used to measure the quantity of water present in soil, as well as moisture/density for quality control in highway construction. 241Am neutron sources are also used in well logging applications, as well as in neutron radiography
Neutron imaging is the process of making an image with neutrons. The resulting image is based on the neutron attenuation properties of the imaged object. The resulting images have much in common with industrial X-ray images, but since the image i ...
, tomography and other radiochemical investigations.
Production of other elements
Americium is a starting material for the production of other transuranic elements and transactinides – for example, 82.7% of 242Am decays to 242Cm and 17.3% to 242Pu. In the nuclear reactor, 242Am is also up-converted by neutron capture to 243Am and 244Am, which transforms by β-decay to 244Cm: :Spectrometer
Americium-241 has been used as a portable source of both gamma rays and alpha particles for a number of medical and industrial uses. The 59.5409 keV gamma ray emissions from 241Am in such sources can be used for indirect analysis of materials in radiography and X-ray fluorescence spectroscopy, as well as for quality control in fixednuclear density gauge
Nuclear densitometry is a technique used in civil construction and the petroleum industry, as well as for mining and archaeology purposes, to measure the density and inner structure of the test material. The processes uses a nuclear density gauge, ...
s and nuclear densometers. For example, the element has been employed to gauge glass thickness to help create flat glass. Americium-241 is also suitable for calibration of gamma-ray spectrometers in the low-energy range, since its spectrum consists of nearly a single peak and negligible Compton continuum (at least three orders of magnitude lower intensity). Americium-241 gamma rays were also used to provide passive diagnosis of thyroid function. This medical application is however obsolete.
Health concerns
As a highly radioactive element, americium and its compounds must be handled only in an appropriate laboratory under special arrangements. Although most americium isotopes predominantly emit alpha particles which can be blocked by thin layers of common materials, many of the daughter products emit gamma-rays and neutrons which have a long penetration depth. If consumed, most of the americium is excreted within a few days, with only 0.05% absorbed in the blood, of which roughly 45% goes to the liver and 45% to the bones, and the remaining 10% is excreted. The uptake to the liver depends on the individual and increases with age. In the bones, americium is first deposited over cortical and trabecular surfaces and slowly redistributes over the bone with time. The biological half-life of 241Am is 50 years in the bones and 20 years in the liver, whereas in thegonad
A gonad, sex gland, or reproductive gland is a mixed gland that produces the gametes and sex hormones of an organism. Female reproductive cells are egg cells, and male reproductive cells are sperm. The male gonad, the testicle, produces sper ...
s (testicles and ovaries) it remains permanently; in all these organs, americium promotes formation of cancer cells as a result of its radioactivity.
Americium often enters landfills from discarded smoke detectors. The rules associated with the disposal of smoke detectors are relaxed in most jurisdictions. In 1994, 17-year-old David Hahn extracted the americium from about 100 smoke detectors in an attempt to build a breeder nuclear reactor. Ken SilversteinThe Radioactive Boy Scout: When a teenager attempts to build a breeder reactor
''
Harper's Magazine
''Harper's Magazine'' is a monthly magazine of literature, politics, culture, finance, and the arts. Launched in New York City in June 1850, it is the oldest continuously published monthly magazine in the U.S. (''Scientific American'' is older, b ...
'', November 1998 There have been a few cases of exposure to americium, the worst case being that of chemical operations technician Harold McCluskey, who at the age of 64 was exposed to 500 times the occupational standard for americium-241 as a result of an explosion in his lab. McCluskey died at the age of 75 of unrelated pre-existing disease.
See also
* Actinides in the environment * :Americium compoundsNotes
References
Bibliography
* * Penneman, R. A. and Keenan T. KThe radiochemistry of americium and curium
University of California, Los Alamos, California, 1960 *
Further reading
* ''Nuclides and Isotopes – 14th Edition'', GE Nuclear Energy, 1989. * *External links
at '' The Periodic Table of Videos'' (University of Nottingham)
ATSDR – Public Health Statement: Americium
{{Authority control Chemical elements Chemical elements with double hexagonal close-packed structure Actinides Carcinogens Synthetic elements