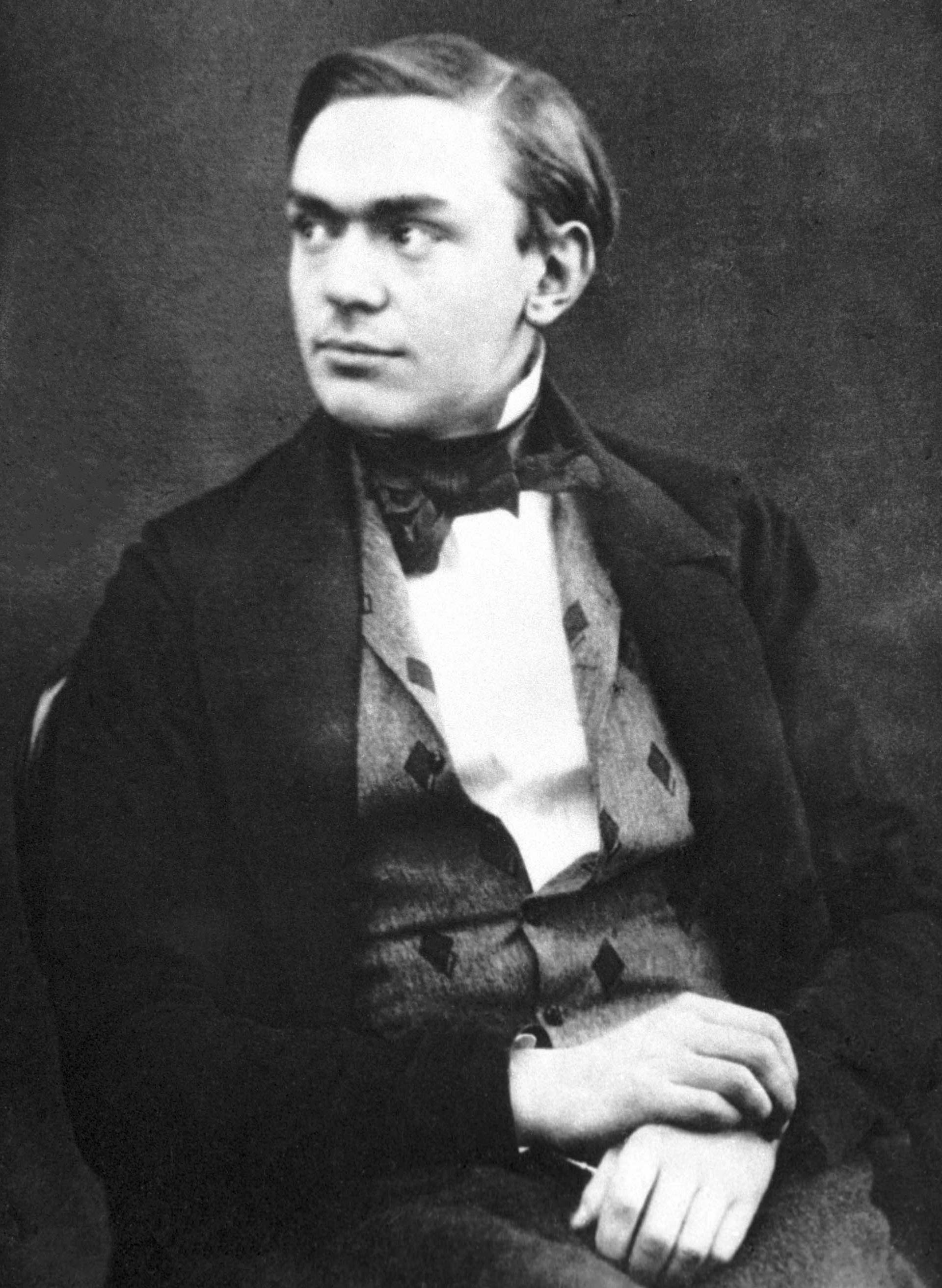|
Nobelium
Nobelium is a synthetic chemical element with the symbol No and atomic number 102. It is named in honor of Alfred Nobel, the inventor of dynamite and benefactor of science. A radioactive metal, it is the tenth transuranic element and is the penultimate member of the actinide series. Like all elements with atomic number over 100, nobelium can only be produced in particle accelerators by bombarding lighter elements with charged particles. A total of twelve nobelium isotopes are known to exist; the most stable is 259No with a half-life of 58 minutes, but the shorter-lived 255No (half-life 3.1 minutes) is most commonly used in chemistry because it can be produced on a larger scale. Chemistry experiments have confirmed that nobelium behaves as a heavier homolog to ytterbium in the periodic table. The chemical properties of nobelium are not completely known: they are mostly only known in aqueous solution. Before nobelium's discovery, it was predicted that it would show a stable +2 oxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actinide Series
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide. The 1985 IUPAC ''Red Book'' recommends that ''actinoid'' be used rather than ''actinide'', since the suffix ''-ide'' normally indicates a negative ion. However, owing to widespread current use, ''actinide'' is still allowed. Since ''actinoid'' literally means ''actinium-like'' (cf. ''humanoid'' or ''android''), it has been argued for semantic reasons that actinium cannot logically be an actinoid, but IUPAC acknowledges its inclusion based on common usage. All the actinides are f-block elements, except the final one (lawrencium) which is a d-block element. Actinium has sometimes been considered d-block instead of lawrencium, but the classi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermium
Fermium is a synthetic element with the symbol Fm and atomic number 100. It is an actinide and the heaviest element that can be formed by neutron bombardment of lighter elements, and hence the last element that can be prepared in macroscopic quantities, although pure fermium metal has not yet been prepared. A total of 19 isotopes are known, with 257Fm being the longest-lived with a half-life of 100.5 days. It was discovered in the debris of the first hydrogen bomb explosion in 1952, and named after Enrico Fermi, one of the pioneers of nuclear physics. Its chemistry is typical for the late actinides, with a preponderance of the +3 oxidation state but also an accessible +2 oxidation state. Owing to the small amounts of produced fermium and all of its isotopes having relatively short half-lives, there are currently no uses for it outside basic scientific research. Discovery Fermium was first discovered in the fallout from the 'Ivy Mike' nuclear test (1 November 1952), the f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Discovery Of The Chemical Elements
The discovery of the 118 chemical elements known to exist as of 2022 is presented in chronological order. The elements are listed generally in the order in which each was first defined as the pure element, as the exact date of discovery of most elements cannot be accurately determined. There are plans to synthesize more elements, and it is not known how many elements are possible. Each element's name, atomic number, year of first report, name of the discoverer, and notes related to the discovery are listed. Periodic table of elements Ancient discoveries Modern discoveries Graphics See also * History of the periodic table * Periodic table * Extended periodic table * ''The Mystery of Matter: Search for the Elements'' (2014/2015 PBS film) * Transfermium Wars References External linksHistory of the Origin of the Chemical Elements and Their DiscoverersLast updated by Boris Pritychenko on March 30, 2004 [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transuranic Element
The transuranium elements (also known as transuranic elements) are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. With the exception of neptunium and plutonium (which have been found in trace amounts in nature), all do not occur naturally on Earth and are synthetic. Overview Of the elements with atomic numbers 1 to 92, most can be found in nature, having stable isotopes (such as hydrogen) or very long-lived radioisotopes (such as uranium), or existing as common decay products of the decay of uranium and thorium (such as radon). The exceptions are elements 43, 61, 85, and 87; all four occur in nature, but only in very minor branches of the uranium and thorium decay chains, and thus all save element 87 were first discovered by synthesis in the laboratory rather than in nature (and even element 87 was discovered from purified samples of its paren ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Element Naming Controversy
The currently accepted names and symbols of the chemical elements are determined by the International Union of Pure and Applied Chemistry (IUPAC), usually following recommendations by the recognized discoverers of each element. However the names of several elements have been the subject of controversies until IUPAC established an official name. In most cases the controversy was due to a priority dispute as to who first found conclusive evidence for the existence of an element, or as to what evidence was in fact conclusive. Element 23 (Vanadium V) Vanadium (named after List of names of Freyja, Vanadís, another name for Freyja, the Scandinavian goddess of fertility) was originally discovery of the chemical elements, discovered by Andrés Manuel del Río (a Spanish-born Mexican mineralogist) in Mexico City in 1801. He discovered the element after being sent a sample of "brown lead" ore (''plomo pardo de Zimapán'', now named vanadinite). Through experimentation, he found it to form s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Element
A synthetic element is one of 24 known chemical elements that do not occur naturally on Earth: they have been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; thus, they are called "synthetic", "artificial", or "man-made". The synthetic elements are those with atomic numbers 95–118, as shown in purple on the accompanying periodic table: these 24 elements were first created between 1944 and 2010. The mechanism for the creation of a synthetic element is to force additional protons into the nucleus of an element with an atomic number lower than 95. All synthetic elements are unstable, but they decay at widely varying rates: the half-lives of their longest-lived isotopes range from microseconds to millions of years. Five more elements that were created artificially are strictly speaking not ''synthetic'' because they were later found in nature in trace quantities: 43Tc, 61Pm, 85At, 93Np, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symbol (chemistry)
Chemical symbols are the abbreviations used in chemistry for chemical elements, functional groups and chemical compounds. Element symbols for chemical elements normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised. History Earlier symbols for chemical elements stem from classical Latin and Greek vocabulary. For some elements, this is because the material was known in ancient times, while for others, the name is a more recent invention. For example, Pb is the symbol for lead (''plumbum'' in Latin); Hg is the symbol for mercury (''hydrargyrum'' in Greek); and He is the symbol for helium (a new Latin name) because helium was not known in ancient Roman times. Some symbols come from other sources, like W for tungsten (''Wolfram'' in German) which was not known in Roman times. A three-letter temporary symbol may be assigned to a newly synthesized (or not yet synthesized) element. For example, "Uno" was the temporary symbol fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alfred Nobel
Alfred Bernhard Nobel ( , ; 21 October 1833 – 10 December 1896) was a Swedes, Swedish chemist, engineer, inventor, businessman, and Philanthropy, philanthropist. He is best known for having bequeathed his fortune to establish the Nobel Prize, though he also made several important contributions to science, holding 355 patents in his lifetime. Nobel's most famous invention was dynamite, a safer and easier means of harnessing the explosive power of nitroglycerin; it was patented in 1867. Nobel displayed an early aptitude for science and learning, particularly in chemistry and languages; he became fluent in six languages and filed his first patent at age 24. He embarked on many business ventures Nobel family, with his family, most notably owning Bofors, an iron and steel producer that he developed into a major manufacturer of cannons and other armaments. Nobel was later inspired to donate his fortune to the Nobel Prize institution, which would annually recognize those who ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha decay ( ), beta decay ( ), and gamma decay ( ), all of which involve emitting one or more particles. The weak force is the mechanism that is responsible for beta decay, while the other two are governed by the electromagnetism and nuclear force. A fourth type of common decay is electron capture, in which an unstable nucleus captures an inner electron from one of the electron shells. The loss of that electron from the shell results in a cascade of electrons dropping down to that lower shell resulting in emission of discrete X-rays from the transitions. A common example is iodine-125 commonly used in medical settings. Radioactive decay is a stochastic (i.e. random) process ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. In an ordinary uncharged atom, the atomic number is also equal to the number of electrons. For an ordinary atom, the sum of the atomic number ''Z'' and the neutron number ''N'' gives the atom's atomic mass number ''A''. Since protons and neutrons have approximately the same mass (and the mass of the electrons is negligible for many purposes) and the mass defect of the nucleon binding is always small compared to the nucleon mass, the atomic mass of any atom, when expressed in unified atomic mass units (making a quantity called the "relative isotopic mass"), is within 1% of the whole number ''A''. Atoms with the same atomic number but dif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as or indicating a helium ion with a +2 charge (missing its two electrons). Once the ion gains electrons from its environment, the alpha particle becomes a normal (electrically neutral) helium atom . Alpha particles have a net spin of zero. Due to the mechanism of their production in standard alpha radioactive decay, alpha particles generally have a kinetic energy of about 5 MeV, and a velocity in the vicinity of 4% of the speed of light. (See discussion below for the limits of these figures in alpha decay.) They are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion-exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, the purification of chemicals and separation of substances. Ion exchange usually describes a process of purification of aqueous solutions using solid polymeric ion-exchange resin. More precisely, the term encompasses a large variety of processes where ions are exchanged between two electrolytes. Aside from its use to purify drinking water, the technique is widely applied for purification and separation of a variety of industrially and medicinally important chemicals. Although the term usually refers to applications of synthetic (man-made) resins, it can include many other materials such as soil. Typical ion exchangers are ion-exchange resins (functionalized porous or gel polymer), zeolites, montmorillonite, clay, and soil humus. Ion exc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |