Alkyne Complexes 1 Allylic Alcohol on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 The
The
2 CH4 + 3/2 O2 -> HC#CH + 3 H2O
Propyne, also industrially useful, is also prepared by
 Via the
Via the
 The largest scale application of this technology is the conversion of acetylene to ethylene in refineries (the steam cracking of alkanes yields a few percent acetylene, which is selectively hydrogenated in the presence of a
The largest scale application of this technology is the conversion of acetylene to ethylene in refineries (the steam cracking of alkanes yields a few percent acetylene, which is selectively hydrogenated in the presence of a RCH=CR'H + H2 -> RCH2CR'H2
The addition of one equivalent of to internal alkynes gives cis-alkenes.
RC#CR' + 2 Br2 -> RCBr2CR'Br2
The addition of nonpolar bonds across is general for silanes, boranes, and related hydrides. The
PhC#CH + H2O -> PhCOCH3
:HC#C(CH2)5C#CH + 2H2O -> CH3CO(CH2)5COCH3
HC#C-CH3 <=> CH2=C=CH2
RC#CR + R'C#CR' <=> 2RC#CR'
Oxidative cleavage of alkynes proceeds via cycloaddition to metal oxides. Most famously,
2CH2O + HC#CH -> HOCH2CCCH2OH
In the  This reactivity exploits the fact that terminal alkynes are weak acids, whose typical p''K''a values around 25 place them between that of
This reactivity exploits the fact that terminal alkynes are weak acids, whose typical p''K''a values around 25 place them between that of RC#CH + MX -> RC#CM + HX
where MX = NaNH2, 2R-\!\!-H -> ce\ce] R-\!\!-\!\!-R
In the
Acetylene
Acetylene ( systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pur ...
Propyne
Propyne (methylacetylene) is an alkyne with the chemical formula . It is a component of MAPD gas—along with its isomer propadiene (allene), which was commonly used in gas welding. Unlike acetylene, propyne can be safely condensed.Peter Pä ...
1-Butyne
1-Butyne is an organic compound with the chemical formula HC2CH2CH3. It is a colorless combustable gas. 1-Butyne participates in reactions typical for terminal alkynes, such as alkyne metathesis, hydrogenation, condensation with formaldehyde. Bas ...
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
, an alkyne is an unsaturated
Saturation, saturated, unsaturation or unsaturated may refer to:
Chemistry
* Saturation, a property of organic compounds referring to carbon-carbon bonds
**Saturated and unsaturated compounds
**Degree of unsaturation
**Saturated fat or fatty acid ...
hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
containing at least one carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes ...
—carbon triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond orde ...
. The simplest acyclic alkynes with only one triple bond and no other functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the res ...
s form a homologous series
In organic chemistry, a homologous series is a sequence of compounds with the same functional group and similar chemical properties in which the members of the series can be branched or unbranched, or differ by molecular formula of and molecu ...
with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne
Acetylene ( systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
using IUPAC nomenclature
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry (IUPAC).
The ...
. Like other hydrocarbons, alkynes are generally hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, ...
.
Structure and bonding
In acetylene, the H–C≡Cbond angle
Bond or bonds may refer to:
Common meanings
* Bond (finance), a type of debt security
* Bail bond, a commercial third-party guarantor of surety bonds in the United States
* Chemical bond, the attraction of atoms, ions or molecules to form chemi ...
s are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometer
The picometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: pm) or picometer ( American spelling) is a unit of length in the International System of Units (SI), equal to , or one trillionth ...
s is much shorter than the C=C distance in alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s (134 pm) or the C–C bond in alkanes (153 pm).
: The
The triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond orde ...
is very strong with a bond strength
In chemistry, bond energy (''BE''), also called the mean bond enthalpy or average bond enthalpy is the measure of bond strength in a chemical bond. IUPAC defines bond energy as the average value of the gas-phase bond-dissociation energy (usually a ...
of 839 kJ/mol. The sigma bond contributes 369 kJ/mol, the first pi bond contributes 268 kJ/mol and the second pi-bond of 202 kJ/mol bond strength. Bonding usually discussed in the context of molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century.
In molecular orbital theory, electrons in a molec ...
, which recognizes the triple bond as arising from overlap of s and p orbitals. In the language of valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
, the carbon atoms in an alkyne bond are sp hybridized
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to f ...
: they each have two unhybridized p orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in a ...
s and two sp hybrid orbitals. Overlap of an sp orbital from each atom forms one sp–sp sigma bond
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools o ...
. Each p orbital on one atom overlaps one on the other atom, forming two pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbita ...
s, giving a total of three bonds. The remaining sp orbital on each atom can form a sigma bond to another atom, for example to hydrogen atoms in the parent acetylene. The two sp orbitals project on opposite sides of the carbon atom.
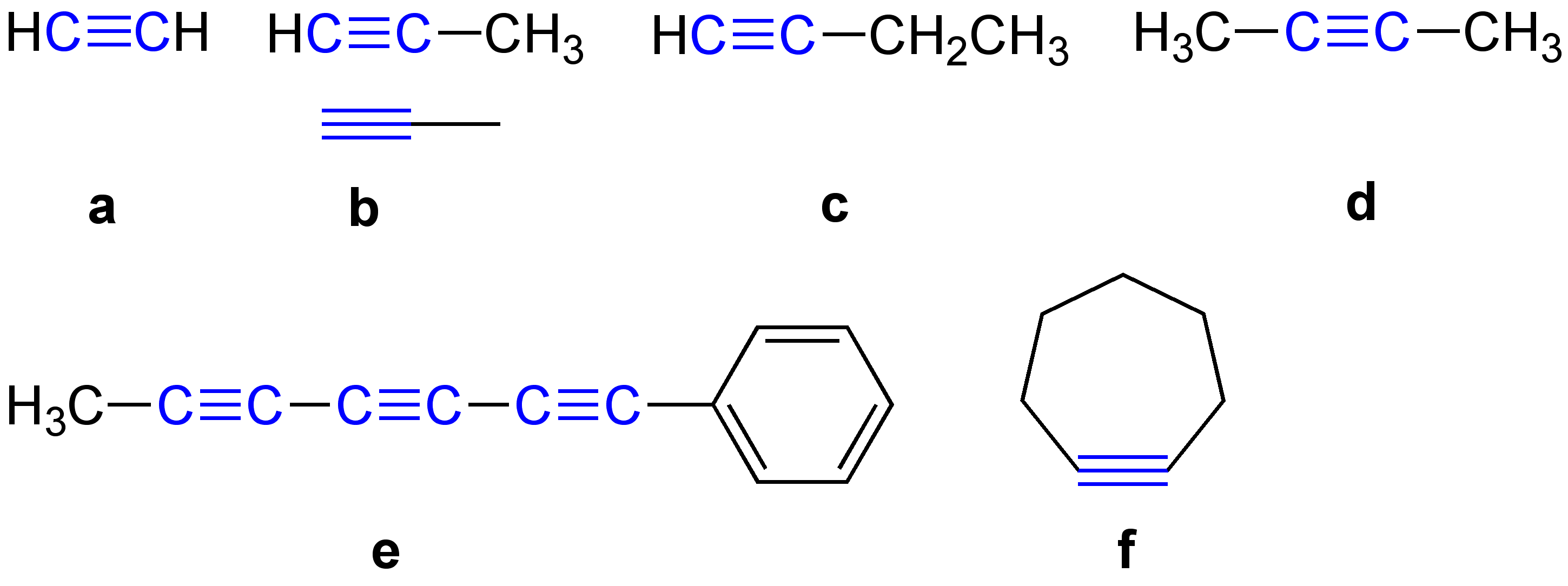
Terminal and internal alkynes
Internal alkynes feature carbon substituents on each acetylenic carbon. Symmetrical examples includediphenylacetylene
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a ligand in organometallic chemistry.
Prepa ...
and 3-hexyne.
Terminal alkynes have the formula . An example is methylacetylene
Propyne (methylacetylene) is an alkyne with the chemical formula . It is a component of MAPD gas—along with its isomer propadiene (allene), which was commonly used in gas welding. Unlike acetylene, propyne can be safely condensed.Peter Pä ...
(propyne using IUPAC nomenclature). Terminal alkynes, like acetylene
Acetylene ( systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pur ...
itself, are mildly acidic, with p''K''a values of around 25. They are far more acidic than alkenes and alkanes, which have p''K''a values of around 40 and 50, respectively. The acidic hydrogen on terminal alkynes can be replaced by a variety of groups resulting in halo-, silyl-, and alkoxoalkynes. The carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3CH ...
s generated by deprotonation of terminal alkynes are called acetylide
In organometallic chemistry, acetylide refers to chemical compounds with the chemical formulas and , where M is a metal. The term is used loosely and can refer to substituted acetylides having the general structure (where R is an organic side ch ...
s.
Naming alkynes
In systematic chemical nomenclature, alkynes are named with the Greek prefix system without any additional letters. Examples include ethyne or octyne. In parent chains with four or more carbons, it is necessary to say where the triple bond is located. Foroctyne
Octynes are alkynes with one triple bond and the molecular formula C8H14.
The isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but disti ...
, one can either write 3-octyne or oct-3-yne when the bond starts at the third carbon. The lowest number possible is given to the triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond orde ...
. When no superior functional groups are present, the parent chain must include the triple bond even if it is not the longest possible carbon chain in the molecule. Ethyne is commonly called by its trivial name acetylene.
In chemistry, the suffix -yne
In chemistry, the suffix -yne is used to denote the presence of a triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds ...
is used to denote the presence of a triple bond. In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
, the suffix often follows IUPAC nomenclature
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry (IUPAC).
The ...
. However, inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemi ...
s featuring unsaturation in the form of triple bonds may be denoted by substitutive nomenclature with the same methods used with alkynes (i.e. the name of the corresponding saturated compound is modified by replacing the "-ane
The suffix -ane in organic chemistry forms the names of organic compounds where the -C-C- group has been attributed the highest priority according to the rules of organic nomenclature. Such organic compounds are called alkanes. They are saturated ...
" ending with "-yne"). "-diyne" is used when there are two triple bonds, and so on. The position of unsaturation is indicated by a numerical locant
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Numeric locants
The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of n ...
immediately preceding the "-yne" suffix, or 'locants' in the case of multiple triple bonds. Locants are chosen so that the numbers are low as possible. "-yne" is also used as an infix
An infix is an affix inserted inside a word stem (an existing word or the core of a family of words). It contrasts with '' adfix,'' a rare term for an affix attached to the outside of a stem, such as a prefix or suffix.
When marking text for i ...
to name substituent groups that are triply bound to the parent compound.
Sometimes a number between hyphen
The hyphen is a punctuation mark used to join words and to separate syllables of a single word. The use of hyphens is called hyphenation. ''Son-in-law'' is an example of a hyphenated word. The hyphen is sometimes confused with dashes ( figur ...
s is inserted before it to state which atoms the triple bond is between. This suffix arose as a collapsed form of the end of the word "acetylene
Acetylene ( systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pur ...
". The final "-e" disappears if it is followed by another suffix that starts with a vowel.
Structural isomerism
Alkynes having four or morecarbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes ...
atoms can form different structural isomer
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term met ...
s by having the triple bond in different positions or having some of the carbon atoms be substituents rather than part of the parent chain. Other non-alkyne structural isomers are also possible.
* : acetylene
Acetylene ( systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pur ...
only
* : propyne
Propyne (methylacetylene) is an alkyne with the chemical formula . It is a component of MAPD gas—along with its isomer propadiene (allene), which was commonly used in gas welding. Unlike acetylene, propyne can be safely condensed.Peter Pä ...
only
* : 2 isomers: 1-butyne
1-Butyne is an organic compound with the chemical formula HC2CH2CH3. It is a colorless combustable gas. 1-Butyne participates in reactions typical for terminal alkynes, such as alkyne metathesis, hydrogenation, condensation with formaldehyde. Bas ...
, and 2-butyne
2-Butyne (dimethylacetylene, crotonylene or but-2-yne) is an alkyne with chemical formula CH3C≡CCH3. Produced artificially, it is a colorless, volatile, pungent liquid at standard temperature and pressure.
2-Butyne is of interest to physical c ...
* : 3 isomers: 1-pentyne, 2-pentyne, and 3-methyl-butyne
* : 7 isomers: 1-hexyne
1-Hexyne (''n''-butylacetylene) is a hydrocarbon consisting of a straight six-carbon chain having a terminal alkyne. Its molecular formula is C6H10. It is a liquid at room temperature that is colorless or pale yellow in appearance.
Reactions
1 ...
, 2-hexyne
2-Hexyne is an organic compound that belongs to the alkyne group. Just like its isomers, it also has the chemical formula of C6H10.
Reactions
2-Hexyne can be semihydrogenated to yield 2-hexene or fully hydrogenated to hexane. With appropriate nob ...
, 3-hexyne, 4-methyl-1-pentyne, 4-methyl-2-pentyne, 3-methyl-1-pentyne, 3,3-dimethyl-1-butyne
Synthesis
Cracking
Commercially, the dominant alkyne is acetylene itself, which is used as a fuel and a precursor to other compounds, e.g.,acrylate
Acrylates (IUPAC: prop-2-enoates) are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion C H2=CHC OO−. Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acr ...
s. Hundreds of millions of kilograms are produced annually by partial oxidation of natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbon ...
:
: thermal cracking
In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking o ...
of hydrocarbons.
Dehydrohalogenation and related reactions
Alkynes are prepared from 1,2- and 1,1-alkyl dihalides by doubledehydrohalogenation
In chemistry, dehydrohalogenation is an elimination reaction which removes a hydrogen halide from a substrate. The reaction is usually associated with the synthesis of alkenes, but it has wider applications.
Dehydrohalogenation from alkyl halid ...
. The reaction provides a means to generate alkynes from alkenes, which are first halogenated and then dehydrohalogenated. For example, phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.
Preparation
In ...
can be generated from styrene by bromination followed by treatment of the resulting of styrene dibromide with sodium amide
Sodium amide, commonly called sodamide (systematic name sodium azanide), is the inorganic compound with the formula . It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is whit ...
in ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogeno ...
:
: Via the
Via the Fritsch–Buttenberg–Wiechell rearrangement
The Fritsch–Buttenberg–Wiechell rearrangement, named for Paul Ernst Moritz Fritsch (1859–1913), Wilhelm Paul Buttenberg, and Heinrich G. Wiechell, is a chemical reaction whereby a 1,1-diaryl-2-bromo-alkene rearranges to a 1,2-diaryl-alkyne b ...
, alkynes are prepared from vinyl bromide
Vinyl bromide is a simple vinyl halide. It is a colorless liquid. It is produced from ethylene dibromide. It is mainly used as a comonomer to confer fire retardant properties to acrylate polymers.
Reactions and applications
It reacts with ...
s. Alkynes can be prepared from aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
s using the Corey–Fuchs reaction
The Corey–Fuchs reaction, also known as the Ramirez–Corey–Fuchs reaction, is a series of chemical reactions designed to transform an aldehyde into an alkyne. The formation of the 1,1-dibromoolefins via phosphine-dibromomethylenes was origina ...
and from aldehydes or ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
s by the Seyferth–Gilbert homologation
The Seyferth–Gilbert homologation is a chemical reaction of an aryl ketone 1 (or aldehyde) with dimethyl (diazomethyl)phosphonate 2 and potassium tert-butoxide to give substituted alkynes 3. Dimethyl (diazomethyl)phosphonate 2 is often called th ...
.
Vinyl chlorides are susceptible to dehydrochlorination. Vinyl chlorides are available from aldehydes using the reagent (chloromethylene)triphenylphosphorane
(Chloromethylene)triphenylphosphorane is the organophosphorus compound with he formula Ph3P=CHCl (Ph = phenyl). It is a white solid but is usually generated and used in situ as a reagent in organic synthesis. It is structurally and chemically re ...
.
Reactions, including applications
Featuring a reactivefunctional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the res ...
, alkynes participate in many organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical rea ...
s. Such use was pioneered by Ralph Raphael
Ralph Alexander Raphael (1 January 1921 – 27 April 1998) was a British organic chemist, well known for his use of acteylene derivatives in the synthesis of natural products with biological activity.
Early life and education
Ralph Raphael w ...
, who in 1955 wrote the first book describing their versatility as intermediates in synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
**Organic synthesis, the chemical synthesis of organi ...
.
Hydrogenation
Being moreunsaturated
Saturation, saturated, unsaturation or unsaturated may refer to:
Chemistry
* Saturation, a property of organic compounds referring to carbon-carbon bonds
**Saturated and unsaturated compounds
**Degree of unsaturation
**Saturated fat or fatty acid ...
than alkenes, alkynes characteristically undergo reactions that show that they are "doubly unsaturated". Alkynes are capable of adding two equivalents of , whereas an alkene adds only one equivalent. Depending on catalysts and conditions, alkynes add one or two equivalents of hydrogen. Partial hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate org ...
, stopping after the addition of only one equivalent to give the alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
, is usually more desirable since alkanes are less useful:
 The largest scale application of this technology is the conversion of acetylene to ethylene in refineries (the steam cracking of alkanes yields a few percent acetylene, which is selectively hydrogenated in the presence of a
The largest scale application of this technology is the conversion of acetylene to ethylene in refineries (the steam cracking of alkanes yields a few percent acetylene, which is selectively hydrogenated in the presence of a palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself nam ...
/silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
catalyst). For more complex alkynes, the Lindlar catalyst
A Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited on calcium carbonate or barium sulfate which is then poisoned with various forms of lead or sulfur. It is used for the hydrogenation of alkynes to alkenes (i.e. ...
is widely recommended to avoid formation of the alkane, for example in the conversion of phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.
Preparation
In ...
to styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
. Similarly, halogenation
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polyme ...
of alkynes gives the alkene dihalides or alkyl tetrahalides:
:
:Addition of halogens and related reagents
Alkynes characteristically are capable of adding two equivalents of halogens and hydrogen halides. :hydroboration
In organic chemistry, hydroboration refers to the addition of a hydrogen- boron bond to certain double and triple bonds involving carbon (, , , and ). This chemical reaction is useful in the organic synthesis of organic compounds.
Hydroboration ...
of alkynes gives vinylic boranes which oxidize to the corresponding aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
or ketone. In the thiol-yne reaction the substrate is a thiol.
Addition of hydrogen halides has long been of interest. In the presence of mercuric chloride
Mercury(II) chloride (or mercury bichloride, mercury dichloride), historically also known as sulema or corrosive sublimate, is the inorganic chemical compound of mercury and chlorine with the formula HgCl2. It is white crystalline solid and is ...
as a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, acetylene and hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride g ...
react to give vinyl chloride
Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC ...
. While this method has been abandoned in the West, it remains the main production method in China.
Hydration
Thehydration reaction
In chemistry, a hydration reaction is a chemical reaction in which a substance combines with water. In organic chemistry, water is added to an unsaturated substrate, which is usually an alkene or an alkyne. This type of reaction is employed indust ...
of acetylene gives acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the ...
. The reaction proceeds by formation of vinyl alcohol
Vinyl alcohol, also called ethenol (IUPAC name; not ethanol), is the simplest enol. With the formula , it is a labile compound that converts to acetaldehyde. It is not a precursor to polyvinyl alcohol.
Synthesis
Vinyl alcohol can be formed by t ...
, which undergoes tautomerizes to form the aldehyde. This reaction was once a major industrial process but it has been displaced by the Wacker process
The Wacker process or the Hoechst-Wacker process (named after the chemical companies of the same name) refers to the oxidation of ethylene to acetaldehyde in the presence of palladium(II) chloride as the catalyst. This chemical reaction was one of ...
. This reaction occurs in nature, the catalyst being acetylene hydratase Acetylene hydratase (, AH) is a bacterial enzyme, originally discovered in the anaerobic microorganism ''Pelobactor acetylenicus'', that catalyzes the non-redox hydration of acetylene to form acetaldehyde
Acetaldehyde (IUPAC systematic name ethan ...
.
The hydration of phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.
Preparation
In ...
gives acetophenone
Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.
Production
Acetophenone is formed as a byproduct of the cumene ...
, and the catalyzed hydration of 1,8-nonadiyne to 2,8-nonanedione:
:Tautomerism
Terminal alkyl alkynes exhibit tautomerism.Propyne
Propyne (methylacetylene) is an alkyne with the chemical formula . It is a component of MAPD gas—along with its isomer propadiene (allene), which was commonly used in gas welding. Unlike acetylene, propyne can be safely condensed.Peter Pä ...
exists in equilibrium with allene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres (). Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which i ...
:
:Cycloadditions and oxidation
Alkynes undergo diversecycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". ...
reactions. The Diels–Alder reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a Conjugated system, conjugated diene and a substituted alkene, commonly termed the Diels–Alder reaction#The dienophile, dienophile, to form a substituted cyclohexe ...
with 1,3-diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature ...
s give 1,4-cyclohexadienes
1,4-Cyclohexadiene is an organic compound with the formula C6H8. It is a colourless, flammable liquid that is of academic interest as a prototype of a large class of related compounds called terpenoids, an example being γ-terpinene. An isomer of ...
. This general reaction has been extensively developed. Electrophilic alkynes are especially effective dienophiles. The "cycloadduct" derived from the addition of alkynes to 2-pyrone eliminates carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
to give the aromatic
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to sat ...
compound. Other specialized cycloadditions include multicomponent reactions such as alkyne trimerisation
In organic chemistry, an alkyne trimerisation is a +2+2nbsp; cycloaddition reaction in which three alkyne units () react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being appl ...
to give aromatic
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to sat ...
compounds and the +2+1cycloaddition of an alkyne, alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
and carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
in the Pauson–Khand reaction
The Pauson–Khand reaction (or PKR or PK-type reaction) is a chemical reaction described as a 2+2+1">/nowiki>2+2+1/nowiki> cycloaddition between an alkyne, an alkene and carbon monoxide to form a α,β-cyclopentenone. Ihsan Ullah Khand (1935-19 ...
. Non-carbon reagents also undergo cyclization, e.g. Azide alkyne Huisgen cycloaddition
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl ...
to give triazole
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial isomerism, depending on the positioning of the nitrogen atoms within th ...
s. Cycloaddition processes involving alkynes are often catalyzed by metals, e.g. enyne metathesis
An enyne metathesis is an organic reaction taking place between an alkyne and an alkene with a metal carbene catalyst forming a butadiene. This reaction is a variation of olefin metathesis.
The general scheme is given by ''scheme 1'':
:
When the ...
and alkyne metathesis
Alkyne metathesis is an organic reaction that entails the redistribution of alkyne chemical bonds. The reaction requires metal catalysts. Mechanistic studies show that the conversion proceeds via the intermediacy of metal alkylidyne complexes. T ...
, which allows the scrambling of carbyne (RC) centers:
:potassium permanganate
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and , an intensely pink to purple solution.
Potassium permanganate is widely used in the c ...
converts alkynes to a pair of carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
s.
Reactions specific for terminal alkynes
Terminal alkynes are readily converted to many derivatives, e.g. by coupling reactions and condensations. Via the condensation with formaldehyde and acetylene is produced butynediol: :Sonogashira reaction
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vin ...
, terminal alkynes are coupled with aryl or vinyl halides:
: This reactivity exploits the fact that terminal alkynes are weak acids, whose typical p''K''a values around 25 place them between that of
This reactivity exploits the fact that terminal alkynes are weak acids, whose typical p''K''a values around 25 place them between that of ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogeno ...
(35) and ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a h ...
(16):
:LiBu
The Libu ( egy, rbw; also transcribed Rebu, Lebu, Lbou, Libou) were an Ancient Libyan tribe of Berber origin, from which the name ''Libya'' derives.
Early history
Their occupation of Ancient Libya is first attested in Egyptian language text ...
, or RMgX.
The reactions of alkynes with certain metal cations, e.g. and also gives acetylides. Thus, few drops of diamminesilver(I) hydroxide () reacts with terminal alkynes signaled by formation of a white precipitate of the silver acetylide. This reactivity is the basis of alkyne coupling reaction A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R ...
s, including the Cadiot–Chodkiewicz coupling
The Cadiot–Chodkiewicz coupling in organic chemistry is a coupling reaction between a terminal alkyne and a haloalkyne catalyzed by a copper(I) salt such as copper(I) bromide and an amine base.Cadiot,
P.; Chodkiewicz, W. In Chemistry of Acetyl ...
, Glaser coupling
The Glaser coupling is a type of coupling reaction. It is by far the oldest acetylenic coupling and is based on cuprous salts like copper(I) chloride or copper(I) bromide and an additional oxidant like oxygen. The base in its original scope is ammo ...
, and the Eglinton coupling
The Glaser coupling is a type of coupling reaction. It is by far the oldest acetylenic coupling and is based on cuprous salts like copper(I) chloride or copper(I) bromide and an additional oxidant like oxygen. The base in its original scope is am ...
:
:Favorskii reaction
The Favorskii reaction is an organic chemistry reaction between an alkyne and a carbonyl group, under basic conditions. The reaction was discovered in the early 1900s by the Russian chemist Alexei Yevgrafovich Favorskii.
When the carbonyl is ...
and in alkynylation
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne () is added to a carbonyl group () to form an α-alkynyl alcohol ().
When the acetylide is formed from acetylene (), the reaction gives an α-ethynyl alcohol. ...
s in general, terminal alkynes add to carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containin ...
compounds to give the hydroxyalkyne
Propargyl alcohol, or 2-propyn-1-ol, is an organic compound with the formula C3H4O. It is the simplest stable alcohol containing an alkyne functional group. Propargyl alcohol is a colorless viscous liquid that is miscible with water and most po ...
.
Metal complexes
Alkynes form complexes with transition metals. Such complexes occur also in metal catalyzed reactions of alkynes such asalkyne trimerization
In organic chemistry, an alkyne trimerisation is a +2+2nbsp; cycloaddition reaction in which three alkyne units () react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being applica ...
. Terminal alkynes, including acetylene itself, react with water to give aldehydes. The transformation typically requires metal catalysts to give this anti-Markovnikov addition result.
Alkynes in nature and medicine
According toFerdinand Bohlmann
Ferdinand Bohlmann ( 28 August 1921 - 23 September 1991) was a German chemist, known for his studies of plant natural products chemistry, especially terpenoids and polyynes.
Life
Bohlmann studied chemistry in Göttingen from 1939 to 1944 . H ...
, the first naturally occurring acetylenic compound, dehydromatricaria ester, was isolated from an ''Artemisia'' species in 1826. In the nearly two centuries that have followed, well over a thousand naturally occurring acetylenes have been discovered and reported. Polyyne
In organic chemistry, a polyyne () is any organic compound with alternating single and triple bonds; that is, a series of consecutive alkynes, with ''n'' greater than 1. These compounds are also called polyacetylenes, especially in the natural ...
s, a subset of this class of natural products, have been isolated from a wide variety of plant species, cultures of higher fungi, bacteria, marine sponges, and corals. Some acids like tariric acid
Tariric acid is an acetylenic fatty acid that can be found in the tallow-wood tree, ''Ximenia americana''.
Léon-Albert Arnaud (1853–1915) was the first scientist to describe the chemical make-up of tariric acid, an extraction from the glucosid ...
contain an alkyne group. Diynes and triynes, species with the linkage RC≡C–C≡CR′ and RC≡C–C≡C–C≡CR′ respectively, occur in certain plants (''Ichthyothere
''Ichthyothere'' is a genus of flowering plants, found in parts of South America (the Amazon) and Central America.
The name ''ichthyothere'' literally translates as ''fish poison''. These plants' active constituent is a chemical called ichthyoth ...
'', ''Chrysanthemum
Chrysanthemums (), sometimes called mums or chrysanths, are flowering plants of the genus ''Chrysanthemum'' in the family Asteraceae. They are native to East Asia and northeastern Europe. Most species originate from East Asia and the center ...
'', ''Cicuta
''Cicuta'', commonly known as water hemlock, is a genus of four species of highly poisonous plants in the family Apiaceae. They are perennial herbaceous plants which grow up to tall, having distinctive small green or white flowers arranged in a ...
'', '' Oenanthe'' and other members of the Asteraceae
The family Asteraceae, alternatively Compositae, consists of over 32,000 known species of flowering plants in over 1,900 genera within the order Asterales. Commonly referred to as the aster, daisy, composite, or sunflower family, Compositae ...
and Apiaceae
Apiaceae or Umbelliferae is a family of mostly aromatic flowering plants named after the type genus '' Apium'' and commonly known as the celery, carrot or parsley family, or simply as umbellifers. It is the 16th-largest family of flowering plant ...
families). Some examples are cicutoxin
Cicutoxin is a naturally-occurring poisonous chemical compound produced by several plants from the family Apiaceae including water hemlock (''Cicuta'' species) and water dropwort (''Oenanthe crocata''). The compound contains polyene, polyyne, ...
, oenanthotoxin
Oenanthotoxin is a toxin extracted from hemlock water-dropwort (''Oenanthe crocata'') and other plants of the genus '' Oenanthe''. It is a central nervous system poison, and acts as a noncompetitive antagonist of the neurotransmitter gamma-aminob ...
, and falcarinol
Falcarinol (also known as carotatoxin or panaxynol) is a natural pesticide and fatty alcohol found in carrots (''Daucus carota''), red ginseng (''Panax ginseng'') and ivy. In carrots, it occurs in a concentration of approximately 2 mg/kg. ...
. These compounds are highly bioactive, e.g. as nematocide
A nematicide is a type of chemical pesticide used to kill plant-parasitic nematodes. Nematicides have tended to be broad-spectrum toxicants possessing high volatility or other properties promoting migration through the soil. Aldicarb (Temik), a c ...
s. 1-Phenylhepta-1,3,5-triyne is illustrative of a naturally occurring triyne.
Alkynes occur in some pharmaceuticals, including the contraceptive noretynodrel
Noretynodrel, or norethynodrel, sold under the brand name Enovid among others, is a progestin medication which was previously used in birth control pills and in the treatment of gynecological disorders but is now no longer marketed. It was availa ...
. A carbon–carbon triple bond is also present in marketed drugs such as the antiretroviral Efavirenz
Efavirenz (EFV), sold under the brand names Sustiva among others, is an antiretroviral medication used to treat and prevent HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needle ...
and the antifungal Terbinafine
Terbinafine, sold under the brand name Lamisil among others, is an antifungal medication used to treat pityriasis versicolor, fungal nail infections, and ringworm including jock itch and athlete's foot. It is either taken by mouth or appli ...
. Molecules called ene-diynes feature a ring containing an alkene ("ene") between two alkyne groups ("diyne"). These compounds, e.g. calicheamicin
The calicheamicins are a class of enediyne antitumor antibiotics derived from the bacterium '' Micromonospora echinospora'', with calicheamicin γ1 being the most notable. It was isolated originally in the mid-1980s from the chalky soil, or "cal ...
, are some of the most aggressive antitumor drugs known, so much so that the ene-diyne subunit is sometimes referred to as a "warhead". Ene-diynes undergo rearrangement via the Bergman cyclization
The Masamune-Bergman cyclization or Masamune-Bergman reaction or Masamune-Bergman cycloaromatization is an organic reaction and more specifically a rearrangement reaction taking place when an enediyne is heated in presence of a suitable hydrogen ...
, generating highly reactive radical intermediates that attack DNA within the tumor.
See also
*-yne
In chemistry, the suffix -yne is used to denote the presence of a triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds ...
* cycloalkyne
In organic chemistry, a cycloalkyne is the cyclic analog of an alkyne (). A cycloalkyne consists of a closed ring of carbon atoms containing one or more triple bonds. Cycloalkynes have a general formula Because of the linear nature of the alkyne ...
References
{{Authority control