Alkylzinc Reagent on:
[Wikipedia]
[Google]
[Amazon]
 Organozinc compounds in organic chemistry contain carbon (C) to zinc (Zn) chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.
Organozinc compounds in organic chemistry contain carbon (C) to zinc (Zn) chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.
The Chemistry of Organozinc Compounds
' (Patai Series
, (Eds. Z. Rappoport and I. Marek), John Wiley & Sons: Chichester, UK, 2006, .''Organozinc reagents – A Practical Approach'', (Eds. P. Knochel and P. Jones), Oxford Medical Publications, Oxford, 1999, . Organozinc compounds were among the first organometallic compounds made. They are less reactive than many other analogous organometallic reagents, such as Grignard and organolithium reagents. In 1848 Edward Frankland prepared the first organozinc compound, diethylzinc, by heating ethyl iodide in the presence of zinc metal.E. Frankland, Liebigs Ann. Chem.,1849, 71, 171 This reaction produced a volatile colorless liquid that spontaneous combusted upon contact with air. Due to their pyrophoric nature, organozinc compounds are generally prepared using air-free techniques. They are unstable toward protic solvents. For many purposes they are prepared in situ, not isolated, but many have been isolated as pure substances and thoroughly characterized. Organozincs can be categorized according to the number of carbon substituents that are bound to the metal. # Diorganozinc (): A class of organozinc compounds in which two alkyl ligands. These may be further divided into subclasses depending on the other
Zinc in organic synthesisOrganozinc Compounds produced by BASF Corporation
{{ChemicalBondsToCarbon
 Organozinc compounds in organic chemistry contain carbon (C) to zinc (Zn) chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.
Organozinc compounds in organic chemistry contain carbon (C) to zinc (Zn) chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.The Chemistry of Organozinc Compounds
' (Patai Series
, (Eds. Z. Rappoport and I. Marek), John Wiley & Sons: Chichester, UK, 2006, .''Organozinc reagents – A Practical Approach'', (Eds. P. Knochel and P. Jones), Oxford Medical Publications, Oxford, 1999, . Organozinc compounds were among the first organometallic compounds made. They are less reactive than many other analogous organometallic reagents, such as Grignard and organolithium reagents. In 1848 Edward Frankland prepared the first organozinc compound, diethylzinc, by heating ethyl iodide in the presence of zinc metal.E. Frankland, Liebigs Ann. Chem.,1849, 71, 171 This reaction produced a volatile colorless liquid that spontaneous combusted upon contact with air. Due to their pyrophoric nature, organozinc compounds are generally prepared using air-free techniques. They are unstable toward protic solvents. For many purposes they are prepared in situ, not isolated, but many have been isolated as pure substances and thoroughly characterized. Organozincs can be categorized according to the number of carbon substituents that are bound to the metal. # Diorganozinc (): A class of organozinc compounds in which two alkyl ligands. These may be further divided into subclasses depending on the other
ligands
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electro ...
attached
# Heteroleptic (RZnX): Compounds which an electronegative or monoanionic ligand (X), such as a halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
, is attached to the zinc center with another alkyl or aryl substituent (R).
# Ionic organozinc compounds: This class is divided into organozincates () and organozinc cations ().
Bonding
In itscoordination complexes
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many m ...
zinc(II) adopts several coordination geometries, commonly octahedral, tetrahedral, and various pentacoordinate geometries. These structural flexibility can be attributed to zinc's electronic configuration rd104s2. The 3d orbital is filled, and therefore, ligand field
Ligand field theory (LFT) describes the bonding, orbital arrangement, and other characteristics of coordination complexes. It represents an application of molecular orbital theory to transition metal complexes. A transition metal ion has nine valen ...
effects are nonexistent. Coordination geometry is thus determined largely by electrostatic and steric interactions. Organozinc compounds usually are two- or three-coordinate, reflecting the strongly donating property of the carbanionic ligands.
Typical diorganozinc complexes have the formula R2Zn. Dialkylzinc compounds are monomeric with a linear coordination at the zinc atom. A polar covalent bond exists between carbon and zinc, being polarized toward carbon due to the differences in electronegativity values (carbon: 2.5 & zinc: 1.65). The dipole moment of symmetric diorganozinc reagents can be seen as zero in these linear complexes, which explains their solubility in nonpolar solvents like cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
. Unlike other binary metal alkyls, the diorganozinc species show a low affinity for complexation with ethereal solvent. Bonding in R2Zn is described as employing sp- hybridized orbitals on Zn.
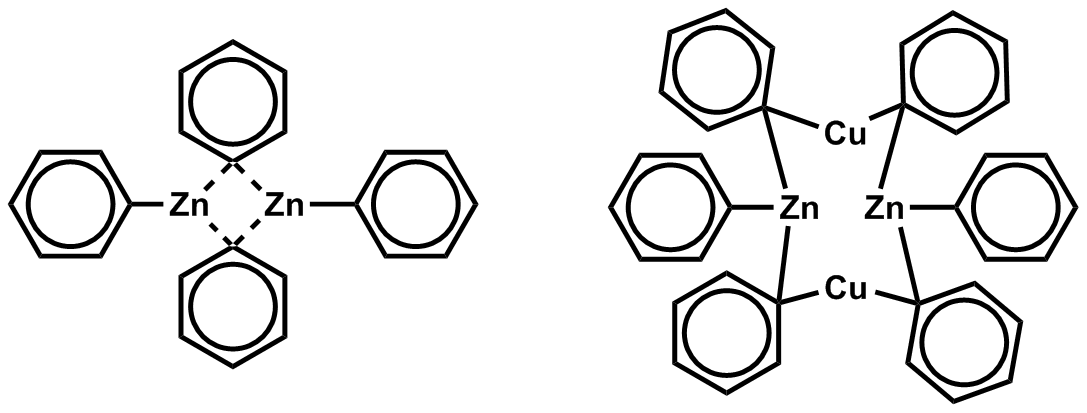
These structures cause zinc to have two bonding d-orbitals and three low-lying non-bonding d-orbitals (see non-bonding orbital), which are available for binding. When zinc lacks electron donating ligands it is unable to obtain coordination saturation, which is a consequence of the large atomic radius and low electron deficiency of zinc. Therefore, it is rare for bridging alkyl or aryl groups to occur due to the weak electron deficiency of the zinc atom. Although, it does occur in some cases such as Ph2Zn (Shown below) and which halogens are the organozinc can form metal clusters (see cluster chemistry). When a halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
ligand is added to the zinc atom both the acceptor and donor character of zinc is enhanced allowing for aggregation.

Synthesis
Several methods exist for the generation of organozinc compounds. Commercially available diorganozinc compounds aredimethylzinc
Dimethylzinc, also known as Zinc methyl, DMZ, or DMZn is a colorless volatile liquid Zn(CH3)2, formed by the action of methyl iodide on zinc at elevated temperature or on zinc sodium alloy.
:2Zn + 2CH3I → Zn(CH3)2 + ZnI2
The sodium assists the ...
, diethylzinc and diphenylzinc. These reagents are expensive and difficult to handle. In one study the active organozinc compound is obtained from much cheaper organobromine precursors:
From zinc metal
Frankland's original synthesis of diethylzinc involves the reaction of ethyl iodide with zinc metal. The zinc must be activated to facilitate this redox reaction. One of such activated form of zinc employed by Frankland is zinc-copper couple. Riecke zinc, produced by in situ reduction of ZnCl2 with potassium, is another activated form of zinc. This form has proven useful for reactions such as Negishi coupling andFukuyama coupling
The Fukuyama coupling is a coupling reaction taking place between a thioester and an organozinc halide in the presence of a palladium catalyst. The reaction product is a ketone. This reaction was discovered by Tohru Fukuyama et al. in 1998. Adv ...
. Formation of organozinc reagents is facilitated for alkyl or aryl halides bearing electron-withdrawing substituents, e.g., nitriles and esters.
Functional group exchange
The two most common zinc functional group interconversion reactions are with halides and boron, which is catalyzed bycopper iodide
Copper(I) iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding.
Copper(I) iodide is white, but samples often appear ...
(CuI) or base. The boron intermediate is synthesized by an initial hydroboration reaction followed by treatment with diethyl zinc. This synthesis shows the utility of organozinc reagents by displaying high selectivity for the most reactive site in the molecule, as well as creating useful coupling partners.
This group transfer reaction can be used in allylation
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic ...
, or other coupling reactions (such as Negishi coupling).
β-Silyl diorganozinc compounds
One of the major drawbacks of diorganozinc alkylations is that only one of the two alkyl substituents is transferred. This problem can be solved by using Me3SiCH2- (TMSM), which is a non-transferable group.Transmetallation
Transmetallation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
is similar to the interconversions displayed above zinc can exchange with other metals such as mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
, lithium, copper, etc. One example of this reaction is the reaction of diphenylmercury
Diphenylmercury is the organomercury compound with the formula Hg(C6H5)2. It is a white solid. The compound is of historic interest as a particularly stable organometallic compound but it finds few uses because of its high toxicity.
Preparation
C ...
with zinc metal to form diphenylzinc
Diphenylzinc is an organozinc compound. It is commonly used as the synthetic equivalent of a Ph− synthon. Solvent-free diphenylzinc exists as dimeric molecules in the solid state.
Diphenylzinc is commercially available. It may be prepar ...
and metallic mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
:
The benefit of transmetalling to zinc it is often more tolerant of other functional groups in the molecule due to the low reactivity which increases selectivity.
*In the synthesis of Maoecrystal V, a directed ortho metalation
Directed ortho metalation (DoM) is an adaptation of electrophilic aromatic substitution in which electrophiles attach themselves exclusively to the ortho- position of a direct metalation group or DMG through the intermediary of an aryllithium com ...
gives the initial aryl-lithium species, which is transmetallated to the desired arylzinc compound. The arylzinc compound is significantly less reactive than the aryl-lithium species and thus better tolerates the functionality in the subsequent coupling with methyl chlorooxaloacetate. Esters are significantly stable against organozinc reagents.
Organozinc can be obtained directly from zinc metal:
:In this method zinc is activated by 1,2-dibromoethane
1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine compound with the chemical formula . Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly synthetic. It is a ...
and trimethylsilyl chloride. A key ingredient is lithium chloride which quickly forms a soluble adduct with the organozinc compound thus removing it from the metal surface.
Reactions
In many of their reactions organozincs appear as intermediates. * In the Frankland–Duppa reaction (1863) an oxalate ester (ROCOCOOR) reacts with an alkyl halide R'X, zinc and hydrochloric acid to the α-hydroxycarboxylic esters RR'COHCOORReformatsky reaction
This organic reaction can be employed to convert α-haloester andketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
or aldehyde to a β-hydroxyester. Acid is needed to protonate the resulting alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
during work up. The initial step is an oxidative addition of zinc metal into the carbon-halogen bond, thus forming a carbon-zinc enolate. This C-Zn enolate can then rearrange to the Oxygen-Zinc enolate via coordination. Once this is formed the other carbonyl containing starting material will coordinate in the manner shown below and give the product after protonation. The benefits of the Reformatsky reaction over the conventional aldol reaction protocols is the following:
# Allows for exceedingly derivatized ketone substrates
# The ester enolate intermediate can be formed in the presence of enolizable moieties
# Well suited for intramolecular reaction Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.
Examples
* intramolecular hydride transfer (transfer of a hy ...
s
Below shows the six-membered transition state of the Zimmerman–Traxler model (Chelation Control, see Aldol reaction), in which R3 is smaller than R4.Kurti, L.; Czako, B. ''Strategic Applications of Named Reactions in Organic Synthesis''; Elsevier: Burlington, 2005.
The Reformatsky reaction has been employed in numerous total syntheses such as the synthesis of C(16),C(18)-bis-epi-cytochalasin D:
The Reformatsky reaction even allows for with zinc homo-enolates. A modification of the Reformatsky reaction is the Blaise reaction.
Simmons–Smith reaction
The Simmons–Smith reagent is used to prepare cyclopropanes from olefin usingmethylene iodide
Diiodomethane or methylene iodide, commonly abbreviated "MI", is an organoiodine compound. Diiodomethane is a colorless liquid; however, it decomposes upon exposure to light liberating iodine, which colours samples brownish. It is slightly solubl ...
as the methylene source. The reaction is effected with zinc. The key zinc-intermediate formed is a carbenoid (iodomethyl)zinc iodide which reacts with alkenes to afford the cyclopropanated product. The rate of forming the active zinc species is increased via ultrasonication since the initial reaction occurs at the surface of the metal.
Although the mechanism has not been fully elaborated it is hypothesized that the organozinc intermediate is a metal- carbenoid. The intermediate is believed to be a three-centered "butterfly-type". This intermediate can be directed by substituents, such as alcohols, to deliver the cyclopropane on the same side of the molecule. Zinc-copper couple is commonly used to activate zinc.
Titanium–zinc methylenation
Organozinc compounds derived from methylene bromide or iodide can electrophilically add to carbonyl groups to form terminal alkenes. The reaction is mechanistically related to the Tebbe reaction and can be catalyzed by various Lewis acids (e.g. TiCl4 or Al2Me6). The reaction is used to introduce deuterium into molecules for isotopic labeling or as an alternative to the Wittig reaction.Negishi coupling
This powerful carbon-carbon bond formingcross-coupling reaction
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = ...
s combines an organic halide and an organozinc halide reagent in the presence of a nickel or palladium catalyst
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
. The organic halide reactant can be alkenyl
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
, aryl, allyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, ...
, or propargyl
In organic chemistry, the propargyl group is a functional group of 2-propynyl with the structure . It is an alkyl group derived from propyne ().
The term propargylic refers to a saturated position ( ''sp''3-hybridized) on a molecular framework n ...
. Alkylzinc coupling with alkyl halides such as bromides and chlorides have also been reported with active catalysts such as Pd-PEPPSI precatalysts, which strongly resist beta-hydride elimination (a common occurrence with alkyl substituents). Either diorganic species or organozinc halides can be used as coupling partners during the transmetallation step in this reaction. Despite the low reactivity of organozinc reagents on organic electrophiles, these reagents are among the most powerful metal nucleophiles toward palladium.
Alkylzinc species require the presence of at least a stoichiometric amount of halide ions in solution to form a "zincate" species of the form RZnX32−, before it can undergo transmetalation to the palladium centre. This behavior contrasts greatly with the case of aryl zinc species. A key step in the catalytic cycle is a transmetalation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
in which a zinc halide exchanges its organic substituent for another halogen with the metal center.
An elegant example of Negishi coupling is Furstner's synthesis of amphidinolide T1:
Fukuyama coupling
Fukuyama coupling
The Fukuyama coupling is a coupling reaction taking place between a thioester and an organozinc halide in the presence of a palladium catalyst. The reaction product is a ketone. This reaction was discovered by Tohru Fukuyama et al. in 1998. Adv ...
is a palladium-catalyzed reaction involving the coupling of an aryl, alkyl, allyl, or α,β- unsaturated thioester
In organic chemistry, thioesters are organosulfur compounds with the functional group . They are analogous to carboxylate esters () with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester, as implied by t ...
compound. This thioester compound can be coupled to a wide range of organozinc reagents in order to reveal the corresponding ketone product. This protocol is useful due to its sensitivity to functional groups such as ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
, acetate
An acetate is a salt (chemistry), salt formed by the combination of acetic acid with a base (e.g. Alkali metal, alkaline, Alkaline earth metal, earthy, Transition metal, metallic, nonmetallic or radical Radical (chemistry), base). "Acetate" als ...
, aromatic halides, and even aldehydes. The chemoselectivity observed indicates ketone formation is more facile than oxidative addition of palladium into these other moieties.
A further example of this coupling method is the synthesis of (+)-biotin
Biotin (or vitamin B7) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name ''biotin'', bor ...
. In this case, the Fukuyama coupling takes place with the thiolactone:
Barbier reaction
The Barbier reaction involvesnucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions di ...
of a carbanion equivalent to a carbonyl. The conversion is similar to the Grignard reaction. The organozinc reagent is generated via an oxidative addition into the alkyl halide. The reaction produces a primary, secondary, or tertiary alcohol via a 1,2-addition
In organic chemistry, an addition reaction is, in simplest terms, an organic reaction where two or more molecules combine to form a larger one (the adduct)..
Addition reactions are limited to chemical compounds that have multiple bonds, such as ...
. The Barbier reaction is advantageous because it is a one-pot process: the organozinc reagent is generated in the presence of the carbonyl substrate. Organozinc reagents are also less water sensitive, thus this reaction can be conducted in water. Similar to the Grignard reaction, a Schlenk equilibrium applies, in which the more reactive dialkylzinc can be formed.
The mechanism resembles the Grignard reaction, in which the metal alkoxide can be generated by a radical stepwise pathway, through single electron transfer, or concerted reaction pathway via a cyclic transition state. An example of this reaction is in Danishefsky's synthesis of cycloproparadicicol. By using the organozinc addition reaction conditions the other functionality of the dienone and the alkyne are tolerated:
Zinc acetylides
The formation of the zinc acetylide proceeds via the intermediacy of a dialkynyl zinc (functional group exchange). Catalytic processes have been developed such as Merck's ephedrine process. Propargylic alcohols can be synthesized from zinc acetylides. These versatile intermediates can then be used for a wide range of chemical transformations such ascross-coupling reaction
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = ...
s, hydrogenation, and pericyclic reactions
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overlap ...
.
In the absence of ligands, the reaction is slow and inefficient. In the presence of chiral ligands, the reaction is fast and gives high conversion. Ryoji Noyori determined that a monozinc-ligand complex is the active species.
Diastereoselectivity for addition of organozinc reagents into aldehydes can be predicted by the following model by Noyori and David A. Evans
David A. Evans (January 11, 1941 – April 29, 2022) was an American chemist who was the Abbott and James Lawrence professor of chemistry at Harvard University. He was a prominent figure in the field of organic chemistry and his research focus ...
:
*The α- stereocenter of the ligand dictates observed stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
of the propargylic alcohols
*The steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
between the aldehyde substituent and the ligand are less important but still dictate the favored conformation
Zinc-acetylides are used in the HIV-1 reverse transcriptase
A reverse transcriptase (RT) is an enzyme used to generate complementary DNA (cDNA) from an RNA template, a process termed reverse transcription. Reverse transcriptases are used by viruses such as HIV and hepatitis B to replicate their genomes, ...
inhibitor Efavirenz
Efavirenz (EFV), sold under the brand names Sustiva among others, is an antiretroviral medication used to treat and prevent HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needlest ...
as well as in Merck's ephedrine derivatives .
Organozincates
The first organozinc ate complex (organozincate) was reported in 1858 byJames Alfred Wanklyn
Prof James Alfred Wanklyn FRSE FCS MRCS (18 February 1834 – 19 July 1906) was a nineteenth-century English analytical chemist who is remembered today chiefly for his "ammonia method" of determining water quality and for his fierce arguments ...
, an assistant to Frankland and concerned the reaction of elemental sodium with diethylzinc:
Organozinc compounds that are strongly Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
ic are vulnerable to nucleophilic attack by alkali metals, such as sodium, and thus form these 'ate compounds'. Two types of organozincates are recognized: tetraorganozincates ( 4Zn2), which are dianionic, and triorganozincates ( 3Zn), which are monoanionic. Their structures, which are determined by the ligands, have been extensively characterized.
Synthesis
Tetraorganozincates such as e4Zni2 can be formed by mixing Me2Zn and MeLi in a 1:2 molar ratio of the rectants. Another example synthetic route to forming spriocyclic organozincates is shown below: Triorganozincates compounds are formed by treating a diorganozinc such as (Me3SiCH2)2Zn with analkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
(K), or an alkali earth metal (Ba, Sr, or Ca). One example is Me3SiCH2)3Zn.
Triethylzincate degrades to sodium hydridoethylzincate(II) as a result of beta-hydride elimination
β-Hydride elimination is a reaction in which an alkyl group bonded to a metal centre is converted into the corresponding metal-bonded hydride and an alkene. The alkyl must have hydrogens on the β-carbon. For instance butyl groups can undergo th ...
:
The product is an edge-shared bitetrahedral structure, with bridging hydride ligands.
Reactions
Although less commonly studied, organozincates often have increased reactivity and selectivity compared to the neutral diorganozinc compounds. They have been useful in stereoselective alkylations of ketones and related carbonyls, ring opening reactions. Aryltrimethylzincates participate in vanadium mediated C-C forming reactions.Organozinc(I) compounds
Low valent organozinc compounds having a Zn–Zn bond are also known. The first such compound,decamethyldizincocene
Decamethyldizincocene is an organozinc compound with the formula
, was reported in 2004.
n2(η5–C5Me5)2 N2 or N-2 may refer to:
* Nitrogen#Allotropes, Dinitrogen (N₂)
Arts and media
* A model number of the Yamaha AvantGrand piano
* "N2", a 2011 song by Japanese indie rock band Asian Kung-Fu Generation, on the album ''Landmark (Asian Kung-Fu Gene ...
It is the first and an unusual example of a compound with a Zn-Zn bond. Decamethyldizincocene is a colorless crystalline solid that burns spontaneously in the pr ...See also
*Compounds of zinc
Zinc compounds are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table. The oxidation state of zinc in most compounds is the group oxidation state of +2. Zinc may be classified as a post-transiti ...
References
External links
Zinc in organic synthesis
{{ChemicalBondsToCarbon